Ann Lab Med.
2019 Nov;39(6):552-560. 10.3343/alm.2019.39.6.552.
Development of Statistical Software for the Korean Laboratory Accreditation Program Using R Language: LaboStats
- Affiliations
-
- 1Department of Laboratory Medicine, The Catholic University of Korea, St. Vincent's Hospital, Suwon, Korea.
- 2Department of Laboratory Medicine, The Catholic University of Korea, Bucheon St. Mary's Hospital, Bucheon, Korea.
- 3Department of Laboratory Medicine, The Catholic University of Korea, Uijeongbu St. Mary's Hospital, Uijeongbu, Korea.
- 4Department of Laboratory Medicine, Soonchunhyang University Bucheon Hospital, Bucheon, Korea.
- 5Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 6Department of Laboratory Medicine, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Korea.
- 7Department of Laboratory Medicine, Korea University College of Medicine, Seoul, Korea.
- 8Department of Laboratory Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 9Department of Laboratory Medicine, Dongguk University Ilsan Hospital, Goyang, Korea.
- 10Department of Laboratory Medicine, The Catholic University of Korea, Eunpyeong St. Mary's Hospital, Seoul, Korea. lyejh@catholic.ac.kr
- KMID: 2450950
- DOI: http://doi.org/10.3343/alm.2019.39.6.552
Abstract
- BACKGROUND
In Korea, the Korean Laboratory Accreditation Program (KLAP) has set minimum standards for verification of clinical test performance. This verification process is time-consuming and labor-intensive when performed manually. We developed a free, statistical software program for KLAP, using the R language (R Foundation for Statistical Computing, Vienna, Austria).
METHODS
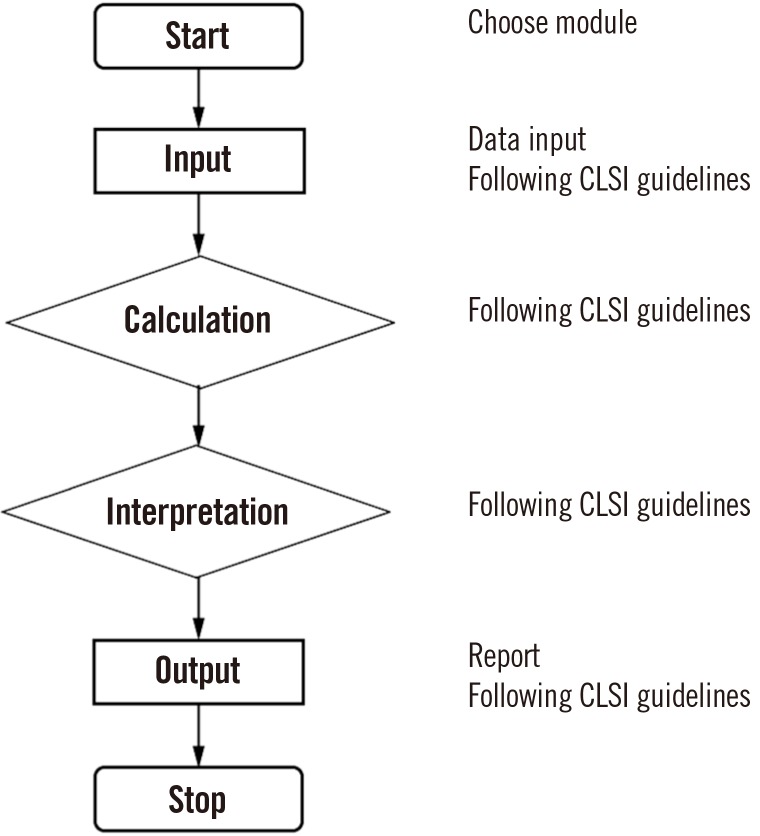
We used CLSI guidelines for the algorithm. We built graphic user interfaces, including data input, with Embarcadero Delphi EX4 (Embarcadero Technologies, Inc., Texas, USA). The R Base Package and MCR Package for Method Comparison Regression were used to implement statistical and graphical procedures.
RESULTS
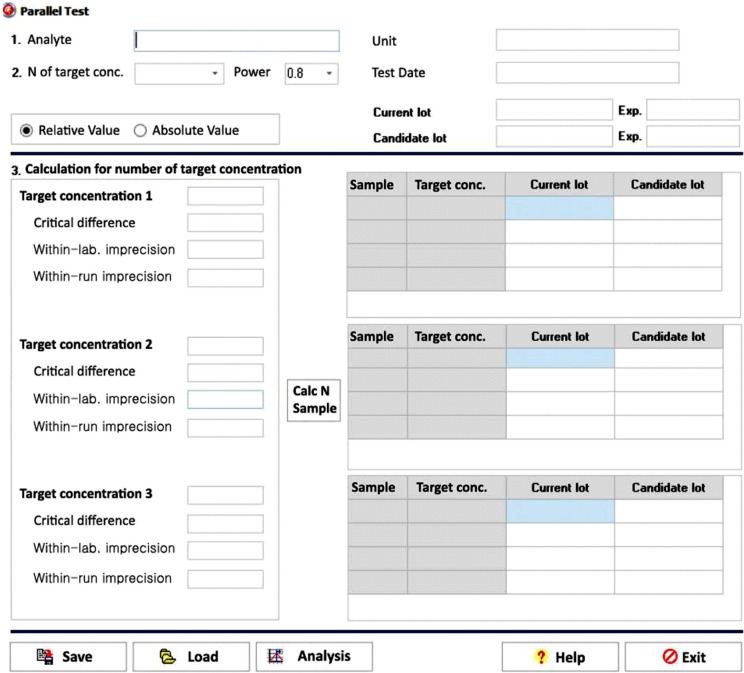
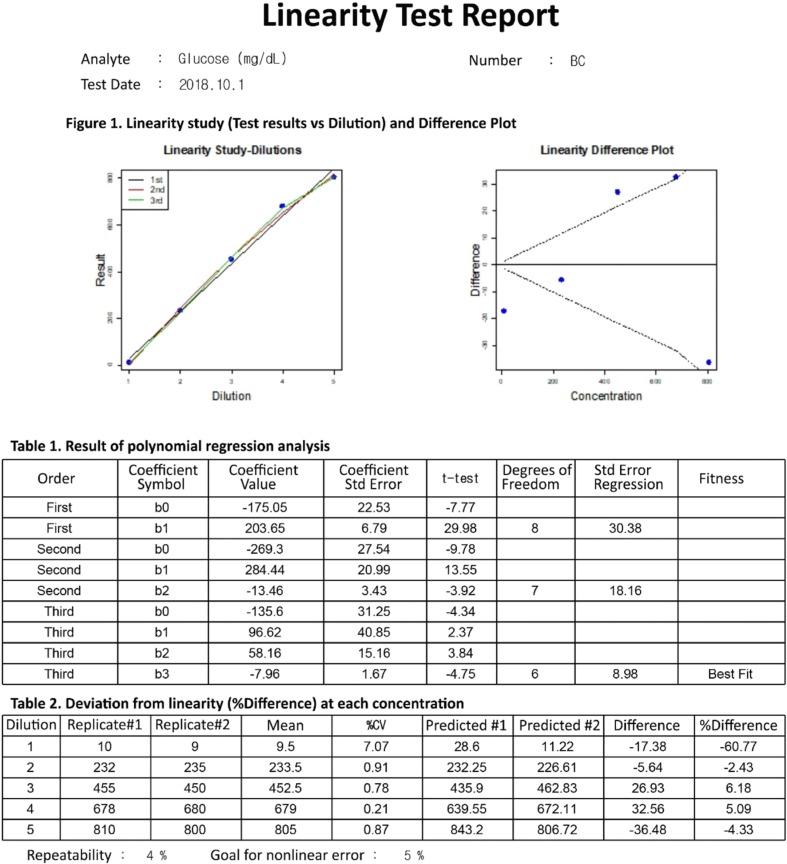
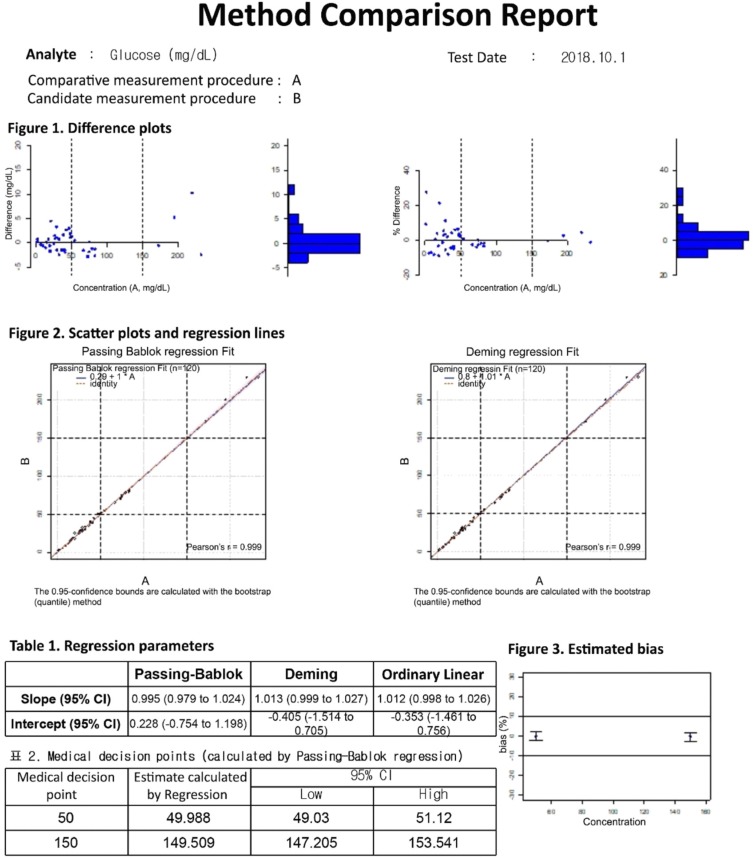
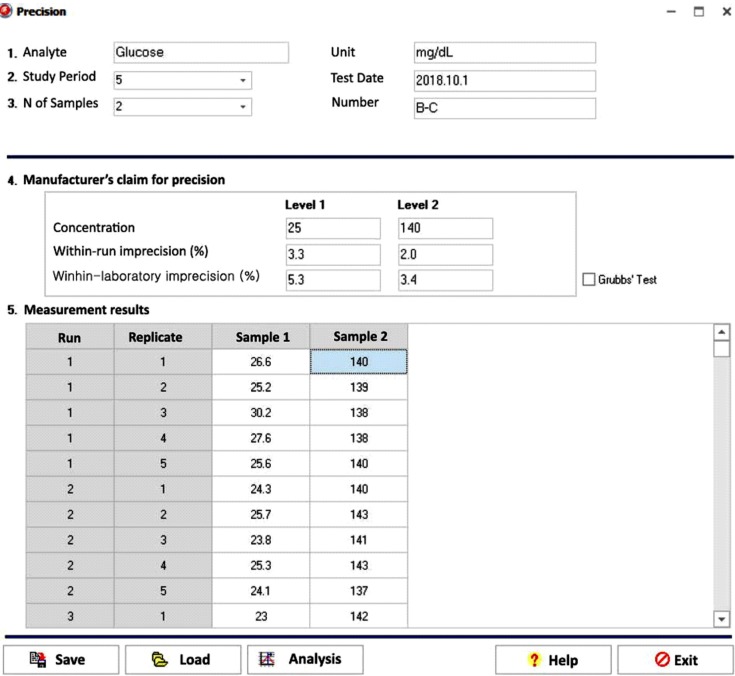
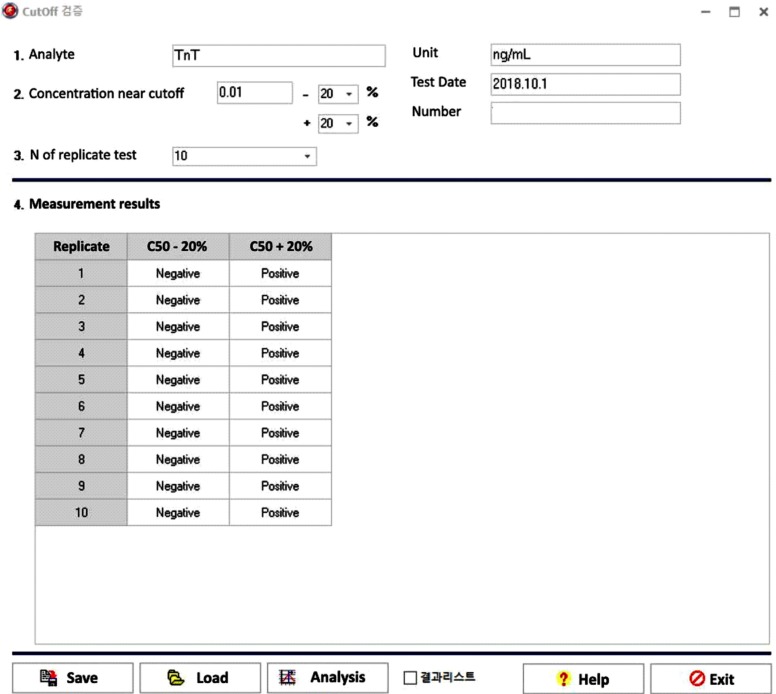
Our program LaboStats has six modules: parallel test, linearity, method comparison, precision, reference interval, and cutoff. Data can be entered into the field either manually or by copying and pasting from an MS Excel worksheet. Users can print out precise reports.
CONCLUSIONS
LaboStats can be useful for evaluating clinical test performance characteristics and preparing documents requested by KLAP.
Figure
Reference
-
1. Guzel O, Guner EI. ISO 15189 accreditation: requirements for quality and competence of medical laboratories, experience of a laboratory I. Clin Biochem. 2009; 42:274–278. PMID: 19863920.2. Linden JV. Errors in transfusion medicine. Scope of the problem. Arch Pathol Lab Med. 1999; 123:563–565. PMID: 10388907.3. Campbell CA, Horvath AR. Harmonization of critical result management in laboratory medicine. Clin Chim Acta. 2014; 432:135–147. PMID: 24246790.4. Ehrmeyer SS. Satisfying regulatory and accreditation requirements for quality control. Clin Lab Med. 2013; 33:27–40. PMID: 23331727.5. Ehrmeyer SS, Laessig RH. Has compliance with CLIA requirements really improved quality in US clinical laboratories? Clin Chim Acta. 2004; 346:37–43. PMID: 15234634.6. Jang MA, Yoon YA, Song J, Kim JH, Min WK, Lee JS, et al. Effect of accreditation on accuracy of diagnostic tests in medical laboratories. Ann Lab Med. 2017; 37:213–222. PMID: 28224767.7. Health Care Financing Administration and Department of Health and Human Services. CFR 493.1255 - standard: calibration and calibration verification procedures. Code of Federal Regulations. Particuology. 2009; 493:42.8. CLSI. User evaluation of between-reagent lot variation. Approved guideline EP26-A. Wayne, PA: Clinical and Laboratory Standards Institute;2013.9. CLSI. Evaluation of the linearity of quantitative measurement procedure: A statistical approach. Approved guideline EP06-A. Wayne, PA: Clinical and Laboratory Standards Institute;2003.10. CLSI. Measurement procedure comparison and bias estimation using patient samples. Approved guideline EP09-A3. Wayne, PA: Clinical and Laboratory Standards Institute;2013.11. CLSI. User verification of precision and estimation of bias. Approved guideline EP15-A3. Wayne, PA: Clinical and Laboratory Standards Institute;2014.12. CLSI. Defining, establishing, and verifying reference intervals in the clinical laboratory. Approved guideline EP28-A3c. Wayne, PA: Clinical and Laboratory Standards Institute;2010.13. CLSI. User protocol for evaluation of qualitative test performance. Approved guideline EP12-A2. Wayne, PA: Clinical and Laboratory Standards Institute;2008.14. Wikipedia. Delphi (IDE). Updated on April 2019. https://en.wikipedia.org/wiki/Delphi_(IDE).15. Rej R, Norton-Wenzel CS. EP evaluator-CLIA (EE-CLIA) for evaluating clinical laboratory methods (Consultant's version with rapid results entry). Clin Chem. 2001; 47:2075–2076.16. Martindale RA, Cembrowski GS, Journault LJ, Crawford JL, Tran C, Hofer TL, et al. Validating new reagents: roadmaps through the wilderness. Lab Med. 2006; 37:347–351.17. Don-Wauchope AC. Lot change for reagents and calibrators. Clin Biochem. 2016; 49:1211–1212. PMID: 27161481.18. Katzman BM, Ness KM, Algeciras-Schimnich A. Evaluation of the CLSI EP26-A protocol for detection of reagent lot-to-lot differences. Clin Biochem. 2017; 50:768–771. PMID: 28322754.19. Algeciras-Schimnich A, Bruns DE, Boyd JC, Bryant SC, La Fortune KA, Grebe SK. Failure of current laboratory protocols to detect lot-to-lot reagent differences: findings and possible solutions. Clin Chem. 2013; 59:1187–1194. PMID: 23592508.20. Glatz JF, Storch J. Unravelling the significance of cellular fatty acid-binding proteins. Curr Opin Lipidol. 2001; 12:267–274. PMID: 11353329.21. Brüggemann L, Quapp W, Wennrich R. Test for non-linearity concerning linear calibrated chemical measurements. Accred Qual Assur. 2006; 11:625–631.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Healthcare Accreditation in Korea: The Current Status and Challenges Ahead
- IMIA Accreditation of Health Informatics Programs
- International Organization for Standardization (ISO) 15189
- Goals and assignments of healthcare accreditation program in Korea
- Improvements during the second cycle healthcare accreditation program in Korea: Toward the global standard for patient safety and quality of healthcare