Investig Clin Urol.
2019 Jul;60(4):235-243. 10.4111/icu.2019.60.4.235.
Establishment of the Seoul National University Prospectively Enrolled Registry for Genitourinary Cancer (SUPER-GUC): A prospective, multidisciplinary, bio-bank linked cohort and research platform
- Affiliations
-
- 1Department of Urology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea. mdrafael@snu.ac.kr
- 2Department of Urology, Inje University Sanggye Paik Hospital, Seoul, Korea.
- 3Department of Urology, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea.
- 4Department of Internal Medicine, Seoul National University Hospital, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 5Department of Radiation Oncology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- 6Department of Radiology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- 7Department of Pathology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- 8Department of Nuclear Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2450540
- DOI: http://doi.org/10.4111/icu.2019.60.4.235
Abstract
- PURPOSE
To establish a prospective, comprehensive, multidisciplinary, bio-bank linked genitourinary cancer cohort based on standard real practice.
MATERIALS AND METHODS
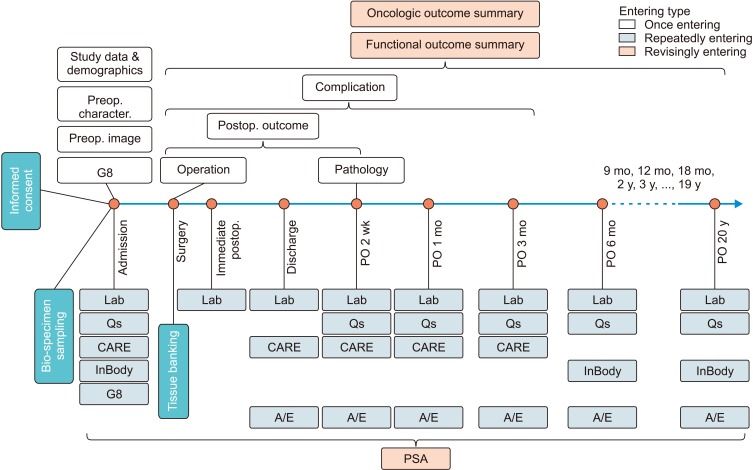
We established the Seoul National University Prospectively Enrolled Registry for Genitourinary Cancer (SUPER-GUC), a prospective cohort clinical database and bio-specimen repository system for prostate cancer (SUPER-PC), renal cell carcinoma (SUPER-RCC), and urothelial cancer (SUPER-UC) at a high-volume, tertiary institution. Each cohort consists of several sub-cohorts based on treatment or disease status. Detailed longitudinal clinical information, and general and disease specific patient-reported outcomes are captured. We use the same evaluation format and questionnaires for all participating departments. Patients' blood, urine, tumor, and normal tissues are collected. The number of registered patients and their basic characteristics are summarized. For the surgical sub-cohort, study participation, bio-specimen, and tissue banking rates are analyzed.
RESULTS
Since March 2016, 11 sub-cohorts for all disease statuses have been opened, ranging from low-risk localized to metastatic disease. SUPER-PC, SUPER-RCC, and SUPER-UC enrolled 929, 796, and 1,221 patients, respectively. Study participation, bio-sampling, and fresh frozen tumor banking rates of surgical sub-cohorts were 89.0% to 93.1%, 91.2% to 99.1%, and 56.9% to 79.1%, respectively.
CONCLUSIONS
SUPER-GUC is a study platform for comparative outcome, quality-of-life, and translational (genetics, biomarkers) research for genitourinary cancer.
MeSH Terms
Figure
Cited by 2 articles
-
Targeted next-generation sequencing for locally advanced prostate cancer in the Korean population
Jungyo Suh, Chang Wook Jeong, Seongmin Choi, Ja Hyeon Ku, Hyeon Hoe Kim, Kwang Soo Kim, Cheol Kwak
Investig Clin Urol. 2020;61(2):127-135. doi: 10.4111/icu.2020.61.2.127.Next-Generation Proteomics–Based Discovery, Verification, and Validation of Urine Biomarkers for Bladder Cancer Diagnosis
Jungyo Suh, Dohyun Han, Ja Hyeon Ku, Hyeon Hoe Kim, Cheol Kwak, Chang Wook Jeong
Cancer Res Treat. 2022;54(3):882-893. doi: 10.4143/crt.2021.642.
Reference
-
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424. PMID: 30207593.
Article2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018; 68:7–30. PMID: 29313949.
Article3. Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol. 2015; 67:519–530. PMID: 25449206.
Article4. Dy GW, Gore JL, Forouzanfar MH, Naghavi M, Fitzmaurice C. Global burden of urologic cancers, 1990-2013. Eur Urol. 2017; 71:437–446. PMID: 28029399.
Article5. Lavallée LT, Fergusson D, Breau RH. The role of randomized controlled trials in evidence-based urology. World J Urol. 2011; 29:257–263. PMID: 21286724.
Article6. Yang W, Zilov A, Soewondo P, Bech OM, Sekkal F, Home PD. Observational studies: going beyond the boundaries of randomized controlled trials. Diabetes Res Clin Pract. 2010; 88(Suppl 1):S3–S9. PMID: 20466165.
Article7. Ahn S, Lee M, Jeong CW. Comparative quality-adjusted survival analysis between radiation therapy alone and radiation with androgen deprivation therapy in patients with locally advanced prostate cancer: a secondary analysis of Radiation Therapy Oncology Group 85-31 with novel decision analysis methods. Prostate Int. 2018; 6:140–144. PMID: 30505816.
Article8. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009; 42:377–381. PMID: 18929686.
Article9. Hollenbeck BK, Dunn RL, Wolf JS Jr, Sanda MG, Wood DP, Gilbert SM, et al. Development and validation of the convalescence and recovery evaluation (CARE) for measuring quality of life after surgery. Qual Life Res. 2008; 17:915–926. PMID: 18574712.
Article10. Bellera CA, Rainfray M, Mathoulin-Pélissier S, Mertens C, Delva F, Fonck M, et al. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol. 2012; 23:2166–2172. PMID: 22250183.
Article11. Decoster L, Van Puyvelde K, Mohile S, Wedding U, Basso U, Colloca G, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations†. Ann Oncol. 2015; 26:288–300. PMID: 24936581.
Article12. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004; 240:205–213. PMID: 15273542.13. Mitropoulos D, Artibani W, Graefen M, Remzi M, Rouprêt M, Truss M. European Association of Urology Guidelines Panel. Reporting and grading of complications after urologic surgical procedures: an ad hoc EAU guidelines panel assessment and recommendations. Eur Urol. 2012; 61:341–349. PMID: 22074761.
Article14. Gibson AL, Holmes JC, Desautels RL, Edmonds LB, Nuudi L. Ability of new octapolar bioimpedance spectroscopy analyzers to predict 4-component-model percentage body fat in Hispanic, Black, and White adults. Am J Clin Nutr. 2008; 87:332–338. PMID: 18258622.
Article15. Maeda K, Koga T, Nasu T, Takaki M, Akagi J. Predictive accuracy of calf circumference measurements to detect decreased skeletal muscle mass and European Society for Clinical Nutrition and Metabolism-defined malnutrition in hospitalized older patients. Ann Nutr Metab. 2017; 71:10–15. PMID: 28647743.
Article16. Koo BK, Kim D, Joo SK, Kim JH, Chang MS, Kim BG, et al. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol. 2017; 66:123–131. PMID: 27599824.
Article17. Ko I, Chang H. Interactive data visualization based on conventional statistical findings for antihypertensive prescriptions using National Health Insurance claims data. Int J Med Inform. 2018; 116:1–8. PMID: 29887229.
Article18. Ko I, Chang H. Interactive visualization of healthcare data using tableau. Healthc Inform Res. 2017; 23:349–354. PMID: 29181247.
Article19. Gandaglia G, Bray F, Cooperberg MR, Karnes RJ, Leveridge MJ, Moretti K, et al. Prostate cancer registries: current status and future directions. Eur Urol. 2016; 69:998–1012. PMID: 26056070.
Article20. Parkin DM. The evolution of the population-based cancer registry. Nat Rev Cancer. 2006; 6:603–612. PMID: 16862191.
Article21. Kang M, Ku JH, Kwak C, Kim HH, Jeong CW. Effects of aspirin, nonsteroidal anti-inflammatory drugs, statin, and COX2 inhibitor on the developments of urological malignancies: a population-based study with 10-year follow-up data in Korea. Cancer Res Treat. 2018; 50:984–991. PMID: 29081218.
Article22. Kim BS, Tae BS, Ku JH, Kwak C, Kim HH, Jeong CW. Rate and association of lower urinary tract infection with recurrence after transurethral resection of bladder tumor. Investig Clin Urol. 2018; 59:10–17.
Article23. Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017; 71:618–629. PMID: 27568654.
Article24. Cooperberg MR, Park S, Carroll PR. Prostate cancer 2004: insights from national disease registries. Oncology (Williston Park). 2004; 18:1239–1247. discussion 1248-50, 1256-8. PMID: 15526829.25. Lubeck DP, Litwin MS, Henning JM, Stier DM, Mazonson P, Fisk R, et al. The CaPSURE database: a methodology for clinical practice and research in prostate cancer. CaPSURE Research Panel. Cancer of the Prostate Strategic Urologic Research Endeavor. Urology. 1996; 48:773–777. PMID: 8911524.26. Nohr EA, Liew Z. How to investigate and adjust for selection bias in cohort studies. Acta Obstet Gynecol Scand. 2018; 97:407–416. PMID: 29415329.
Article27. Törner A, Dickman P, Duberg AS, Kristinsson S, Landgren O, Björkholm M, et al. A method to visualize and adjust for selection bias in prevalent cohort studies. Am J Epidemiol. 2011; 174:969–976. PMID: 21920949.28. Barocas DA, Alvarez J, Resnick MJ, Koyama T, Hoffman KE, Tyson MD, et al. Association between radiation therapy, surgery, or observation for localized prostate cancer and patient-reported outcomes after 3 years. JAMA. 2017; 317:1126–1140. PMID: 28324093.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Optimization of Inflammatory Bowel Disease Cohort Studies in Asia
- Understandings and Key Elements of Trauma Data Bank System
- Primary registry of the WHO International Clinical Trial Registry Platform: Clinical Research Information Service (CRIS)
- Comparison of Characteristics and Survival between Prospective and Retrospective Korea Human Immunodeficiency Virus/Acquired Immune Deficiency Syndrome Cohort Studies
- Cancer screenee cohort study of the National Cancer Center in South Korea