Korean Circ J.
2019 Jul;49(7):629-639. 10.4070/kcj.2018.0446.
Development and Validation of Deep-Learning Algorithm for Electrocardiography-Based Heart Failure Identification
- Affiliations
-
- 1Department of Emergency Medicine, Mediplex Sejong Hospital, Incheon, Korea.
- 2Division of Cardiology, Department of Internal Medicine, Cardiovascular Center, Mediplex Sejong Hospital, Incheon, Korea. learnbyliving9@gmail.com
- KMID: 2450395
- DOI: http://doi.org/10.4070/kcj.2018.0446
Abstract
- BACKGROUND AND OBJECTIVES
Screening and early diagnosis for heart failure (HF) are critical. However, conventional screening diagnostic methods have limitations, and electrocardiography (ECG)-based HF identification may be helpful. This study aimed to develop and validate a deep-learning algorithm for ECG-based HF identification (DEHF).
METHODS
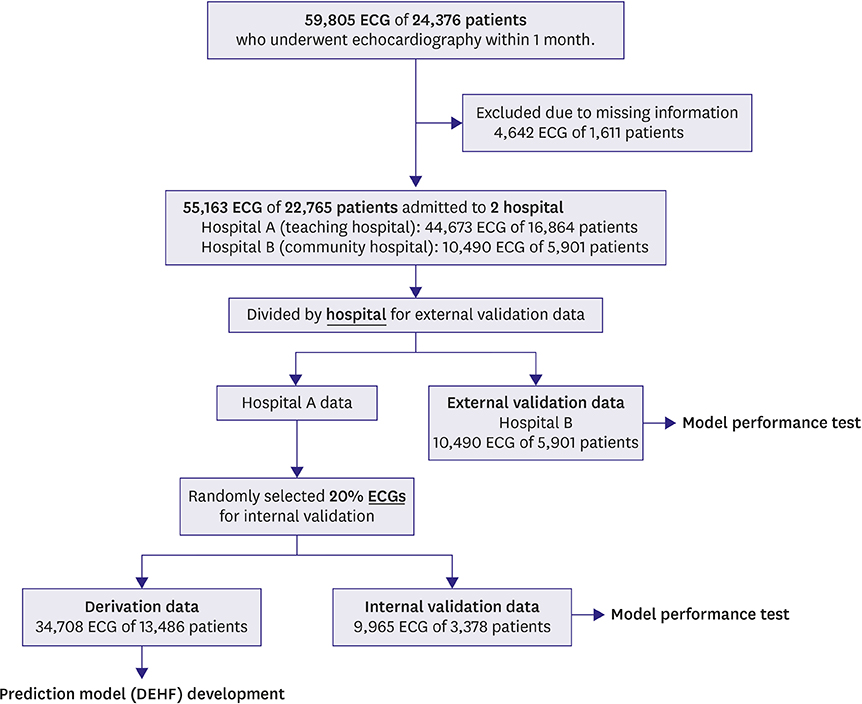
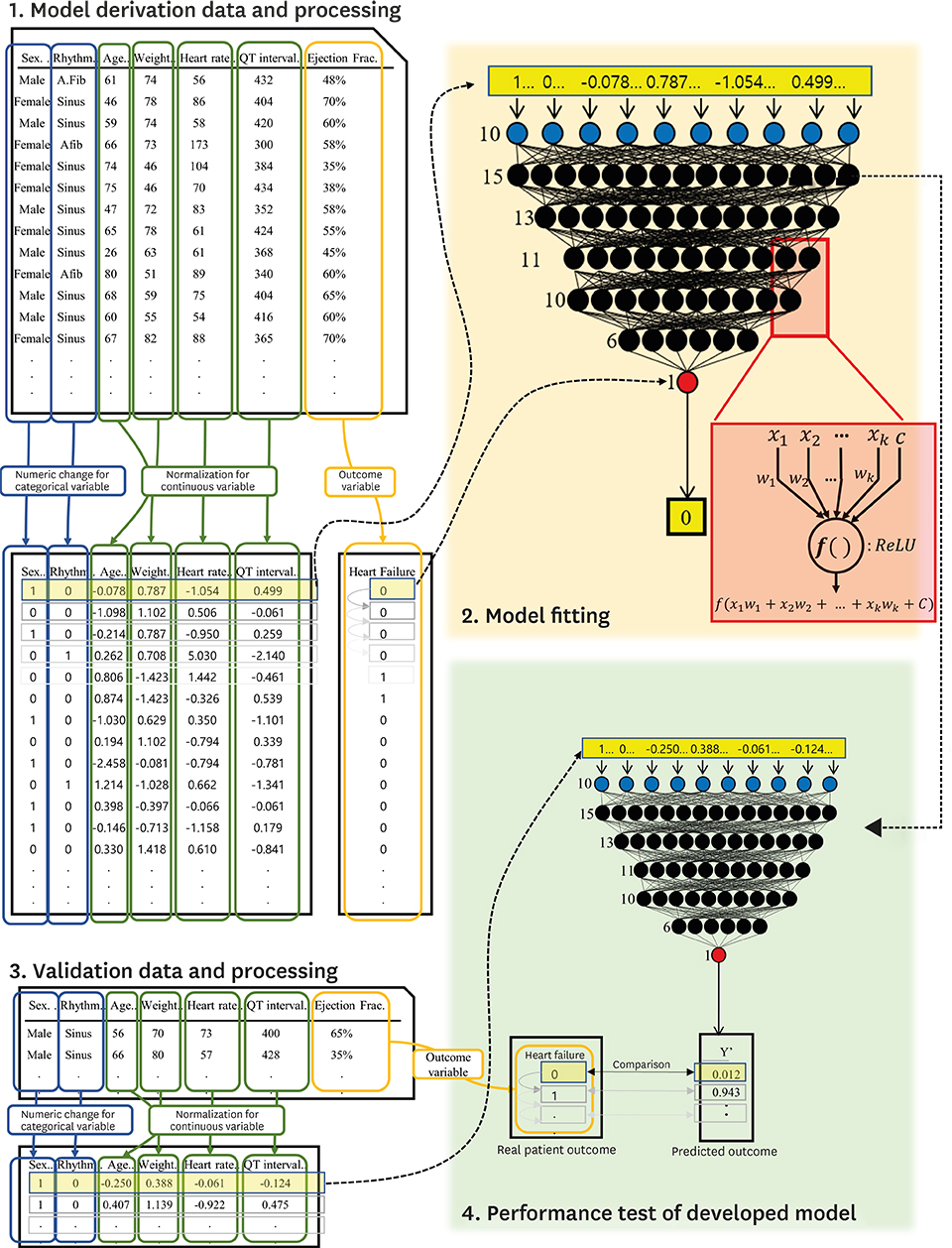
The study involved 2 hospitals and 55,163 ECGs of 22,765 patients who performed echocardiography within 4 weeks were study subjects. ECGs were divided into derivation and validation data. Demographic and ECG features were used as predictive variables. The primary endpoint was detection of HF with reduced ejection fraction (HFrEF; ejection fraction [EF]≤40%), and the secondary endpoint was HF with mid-range to reduced EF (≤50%). We developed the DEHF using derivation data and the algorithm representing the risk of HF between 0 and 1. We confirmed accuracy and compared logistic regression (LR) and random forest (RF) analyses using validation data.
RESULTS
The area under the receiver operating characteristic curves (AUROCs) of DEHF for identification of HFrEF were 0.843 (95% confidence interval, 0.840-0.845) and 0.889 (0.887-0.891) for internal and external validation, respectively, and these results significantly outperformed those of LR (0.800 [0.797-0.803], 0.847 [0.844-0.850]) and RF (0.807 [0.804-0.810], 0.853 [0.850-0.855]) analyses. The AUROCs of deep learning for identification of the secondary endpoint was 0.821 (0.819-0.823) and 0.850 (0.848-0.852) for internal and external validation, respectively, and these results significantly outperformed those of LR and RF.
CONCLUSIONS
The deep-learning algorithm accurately identified HF using ECG features and outperformed other machine-learning methods.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Can artificial Intelligence Prediction Algorithms Exceed Statistical Predictions?
Junbeom Park
Korean Circ J. 2019;49(7):640-641. doi: 10.4070/kcj.2019.0110.Feasibility of fully automated classification of whole slide images based on deep learning
Kyung-Ok Cho, Sung Hak Lee, Hyun-Jong Jang
Korean J Physiol Pharmacol. 2020;24(1):89-99. doi: 10.4196/kjpp.2020.24.1.89.
Reference
-
1. Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016; 13:368–378.2. Ponikowski P, Anker SD, AlHabib KF, et al. Heart failure: preventing disease and death worldwide. ESC Heart Fail. 2014; 1:4–25.3. Ambrosy AP, Fonarow GC, Butler J, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014; 63:1123–1133.4. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017; 70:776–803.5. Bagley SC, White H, Golomb BA. Logistic regression in the medical literature: standards for use and reporting, with particular attention to one medical domain. J Clin Epidemiol. 2001; 54:979–985.6. Breiman L. Statistical modeling: the two cultures. Stat Sci. 2001; 16:199–215.7. Nainwal A, Kumar Y, Jha B. Morphological changes in congestive heart failure ECG. In : 2nd International Conference on Advances in Computing, Communication, & Automation (ICACCA); 2016 Sep 30–Oct 1; Fri, India. Bareilly: Institute of Electrical and Electronics Engineers;2016.8. Hendry PB, Krisdinarti L, Erika M. Scoring system based on electrocardiogram features to predict the type of heart failure in patients with chronic heart failure. Cardiol Res. 2016; 7:110–116.9. Attia ZI, Kapa S, Lopez-Jimenez F, et al. Screening for cardiac contractile dysfunction using an artificial intelligence-enabled electrocardiogram. Nat Med. 2019; 25:70–74.10. Sengupta PP, Kulkarni H, Narula J. Prediction of abnormal myocardial relaxation from signal processed surface ECG. J Am Coll Cardiol. 2018; 71:1650–1660.11. Johnson KW, Torres Soto J, Glicksberg BS, et al. Artificial intelligence in cardiology. J Am Coll Cardiol. 2018; 71:2668–2679.12. Ting DS, Cheung CY, Lim G, et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA. 2017; 318:2211–2223.13. Kwon JM, Lee Y, Lee Y, Lee S, Park J. An algorithm based on deep learning for predicting in-hospital cardiac arrest. J Am Heart Assoc. 2018; 7:e008678.14. LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015; 521:436–444.15. Pal SK, Mitra S. Multilayer perceptron, fuzzy sets, and classification. IEEE Trans Neural Netw. 1992; 3:683–697.16. Nair V, Hinton GE. Rectified linear units improve restricted Boltzmann machines. In : Proceedings of the 27th International Conference on Machine Learning (ICML-10); 2010 Jun 21–24; Mon, Israel. Haifa: International Machine Learning Society;2010.17. Abadi M, Barham P, Chen J, et al. TensorFlow: a system for large-scale machine learning. In : Proceedings of the 12th USENIX Symposium on Operating Systems Design and Implementation (OSDI' 16); 2016 Nov 2–4; Wen, USA. Savannah (GA): USENIX Association;2016.18. Jayalakshmi T, Santhakumaran A. Statistical normalization and backpropagation for classification. Int J Comput Theory Eng. 2011; 3:89–93.19. Shouval R, Hadanny A, Shlomo N, et al. Machine learning for prediction of 30-day mortality after ST elevation myocardial infraction: an acute coronary syndrome Israeli survey data mining study. Int J Cardiol. 2017; 246:7–13.
Article20. Calcagno V, de Mazancourt C. Glmulti: an R package for easy automated model selection with (generalized) linear models. J Stat Softw. 2010; 34:1–29.
Article21. Khalilia M, Chakraborty S, Popescu M. Predicting disease risks from highly imbalanced data using random forest. BMC Med Inform Decis Mak. 2011; 11:51.
Article22. Carpenter J, Bithell J. Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Stat Med. 2000; 19:1141–1164.
Article23. Son CS, Kim YN, Kim HS, Park HS, Kim MS. Decision-making model for early diagnosis of congestive heart failure using rough set and decision tree approaches. J Biomed Inform. 2012; 45:999–1008.
Article24. Masetic Z, Subasi A. Congestive heart failure detection using random forest classifier. Comput Methods Programs Biomed. 2016; 130:54–64.
Article25. Alonso-Betanzos A, Bolón-Canedo V, Heyndrickx GR, Kerkhof PL. Exploring guidelines for classification of major heart failure subtypes by using machine learning. Clin Med Insights Cardiol. 2015; 9:57–71.
Article26. Isler Y. Discrimination of systolic and diastolic dysfunctions using multi-layer perceptron in heart rate variability analysis. Comput Biol Med. 2016; 76:113–119.
Article27. Melillo P, De Luca N, Bracale M, Pecchia L. Classification tree for risk assessment in patients suffering from congestive heart failure via long-term heart rate variability. IEEE J Biomed Health Inform. 2013; 17:727–733.
Article28. Guidi G, Pettenati MC, Melillo P, Iadanza E. A machine learning system to improve heart failure patient assistance. IEEE J Biomed Health Inform. 2014; 18:1750–1756.
Article29. Fong RC, Vedaldi A. Interpretable explanations of black boxes by meaningful perturbation. Proc IEEE Int Conf Comput Vis. 2017; 3449–3457.
Article30. Wolpert DH. The supervised learning no-free-lunch theorems. In : Roy R, Köppen M, Ovaska S, Furuhashi T, Hoffmann F, editors. Soft Computing and Industry. London: Springer;2002. p. 25–42.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Latest Trends in the Use of Deep Learning in Radiology Illustrated Through the Stages of Deep Learning Algorithm Development
- Development of an Optimized Deep Learning Model for Medical Imaging
- Acne Severity Scoring Using Deep Learning
- Deep Learning in the Medical Domain: Predicting Cardiac Arrest Using Deep Learning
- Diagnostic Performance of a New Convolutional Neural Network Algorithm for Detecting Developmental Dysplasia of the Hip on Anteroposterior Radiographs