Yonsei Med J.
2019 Apr;60(4):375-380. 10.3349/ymj.2019.60.4.375.
A Feasibility Study for Diagnosis of Latent Tuberculosis Infection Using an IGRA Point-of-Care Platform in South Korea
- Affiliations
-
- 1Institute for Immunology and Immunological Diseases, Yonsei University College of Medicine, Seoul, Korea. hur1225@gmail.com
- 2Department of Pulmonary and Critical Care Medicine, Hallym University Medical Center, Chuncheon, Korea.
- 3Boditech Med Inc., Chuncheon, Korea.
- 4Department of Immunology and Infection, London School of Hygiene and Tropical Medicine, London, UK.
- KMID: 2450193
- DOI: http://doi.org/10.3349/ymj.2019.60.4.375
Abstract
- PURPOSE
This study aimed to evaluate ichroma™ IGRA-TB, a novel point-of-care platform for assaying IFN-γ release, and to compare it with QuantiFERON-TB Gold In-Tube (QFT-GIT) for identifying Mycobacterium tuberculosis (M. tb) infection.
MATERIALS AND METHODS
We recruited 60 healthy subjects, and blood samples were obtained in QFT-GIT blood collection tubes. The blood collection tubes were incubated at 37℃, and culture supernatant was harvested after 18-24 hours. IFN-γ responses were assessed by the ichroma™ IGRA-TB cartridge and the QFT-GIT IFN-γ enzyme-linked immunosorbent assay. Three active TB patients were recruited as a positive control for M. tb infection.
RESULTS
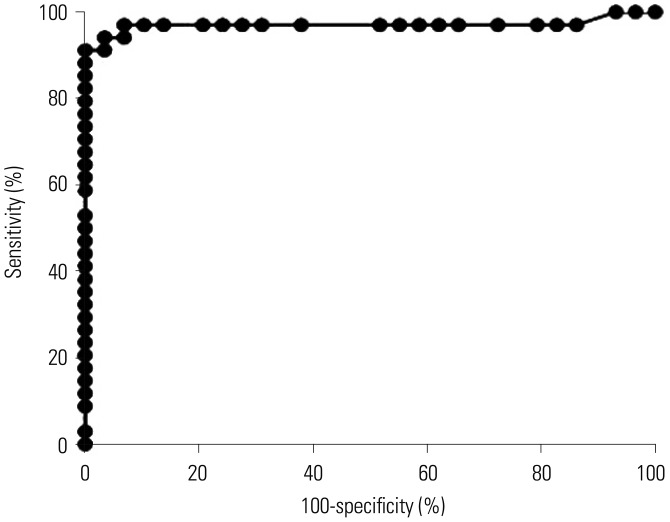
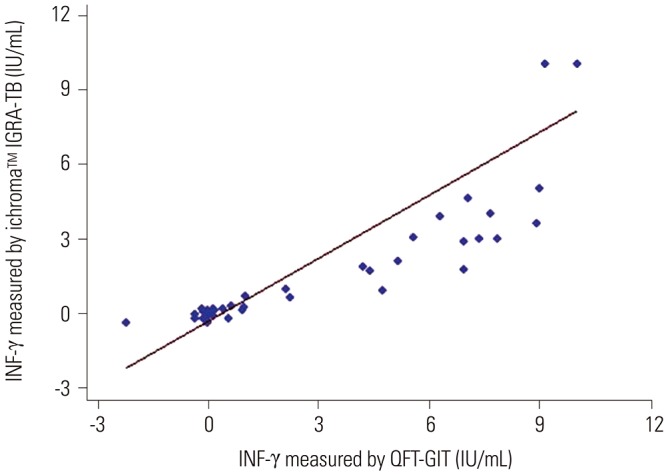
The area under the receiver operating characteristic curve of the ichroma™ IGRA-TB test for differentiating between infected and non-infected individuals was 0.9706 (p < 0.001). Inconsistent positivity between the two tests was found in three participants who showed weak positive IFN-γ responses ( < 1.0 IU/mL) with QFT-GIT. However, the two tests had excellent agreement (95.2%, κ=0.91, p < 0.001), and a very strong positive correlation was observed between the IFN-γ values of both tests (r=0.91, p < 0.001).
CONCLUSION
The diagnostic accuracy demonstrated in this study indicates that the ichromaâ„¢ IGRA-TB test could be used as a rapid diagnostic method for detecting latent TB infection. It may be particularly beneficial in resource-limited places that require cost-effective laboratory diagnostics.
Keyword
MeSH Terms
Figure
Reference
-
1. World Health Organization. Global tuberculosis report 2017. accessed on 2018 April 4. Available at: http://apps.who.int/iris/bitstream/handle/10665/259366/9789241565516-eng.pdf?sequence=1.2. Nahid P, Pai M, Hopewell PC. Advances in the diagnosis and treatment of tuberculosis. Proc Am Thorac Soc. 2006; 3:103–110. PMID: 16493157.3. Pai M, Riley LW, Colford JM Jr. Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis. 2004; 4:761–776. PMID: 15567126.4. World Health Organization. Guidelines on the management of latent tuberculosis infection. accessed on 2018 April 4. Available at: https://apps.who.int/iris/bitstream/handle/10665/136471/9789241548908_eng.pdf;jsessionid=7C6264841AD2EA15619EDBBC84BB7C14?sequence=1.5. Dheda K, Ruhwald M, Theron G, Peter J, Yam WC. Point-of-care diagnosis of tuberculosis: past, present and future. Respirology. 2013; 18:217–232. PMID: 23190246.
Article6. Gonzalez JM, Francis B, Burda S, Hess K, Behera D, Gupta D, et al. Development of a POC test for TB based on multiple immunodominant epitopes of M. tuberculosis specific cell-wall proteins. PLoS One. 2014; 9:e106279. PMID: 25247820.
Article7. Mani V, Paleja B, Larbi K, Kumar P, Tay JA, Siew JY, et al. Microchip-based ultrafast serodiagnostic assay for tuberculosis. Sci Rep. 2016; 6:35845. PMID: 27775039.
Article8. Sahle SN, Asress DT, Tullu KD, Weldemariam AG, Tola HH, Awas YA, et al. Performance of point-of-care urine test in diagnosing tuberculosis suspects with and without HIV infection in selected peripheral health settings of Addis Ababa, Ethiopia. BMC Res Notes. 2017; 10:74. PMID: 28137314.
Article9. Ruhwald M, Aggerbeck H, Gallardo RV, Hoff ST, Villate JI, Borregaard B, et al. Safety and efficacy of the C-Tb skin test to diagnose Mycobacterium tuberculosis infection, compared with an interferon γ release assay and the tuberculin skin test: a phase 3, doubleblind, randomised, controlled trial. Lancet Respir Med. 2017; 5:259–268. PMID: 28159608.
Article10. Bibova I, Linhartova I, Stanek O, Rusnakova V, Kubista M, Suchanek M, et al. Detection of immune cell response to M. tuberculosis-specific antigens by quantitative polymerase chain reaction. Diagn Microbiol Infect Dis. 2012; 72:68–78. PMID: 22085772.
Article11. Kasprowicz VO, Mitchell JE, Chetty S, Govender P, Huang KH, Fletcher HA, et al. A molecular assay for sensitive detection of pathogen-specific T-cells. PLoS One. 2011; 6:e20606. PMID: 21853018.
Article12. Pai NP, Vadnais C, Denkinger C, Engel N, Pai M. Point-of-care testing for infectious diseases: diversity, complexity, and barriers in low- and middle-income countries. PLoS Med. 2012; 9:e1001306. PMID: 22973183.
Article13. World Health Organization. Tuberculosis country profiles. Republic of Korea: accessed on 2018 April 4. Available at: http://www.who.int/tb/country/data/profiles/en/.14. Glynn JR, Whiteley J, Bifani PJ, Kremer K, van Soolingen D. Worldwide occurrence of Beijing/W strains of Mycobacterium tuberculosis: a systematic review. Emerg Infect Dis. 2002; 8:843–849. PMID: 12141971.15. Kim SJ, Bai GH, Lee H, Kim HJ, Lew WJ, Park YK, et al. Transmission of Mycobacterium tuberculosis among high school students in Korea. Int J Tuberc Lung Dis. 2001; 5:824–830. PMID: 11573893.16. Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005; 47:458–472. PMID: 16161804.
Article17. Trikalinos TA, Balion CM. Chapter 9: options for summarizing medical test performance in the absence of a “gold standard. ” J Gen Intern Med. 2012; 27(Suppl 1):S67–S75. PMID: 22648677.
Article18. Metcalfe JZ, Cattamanchi A, McCulloch CE, Lew JD, Ha NP, Graviss EA. Test variability of the QuantiFERON-TB gold in-tube assay in clinical practice. Am J Respir Crit Care Med. 2013; 187:206–211. PMID: 23103734.
Article19. Jonsson J, Westman A, Bruchfeld J, Sturegård E, Gaines H, Schön T. A borderline range for Quantiferon Gold In-Tube results. PLoS One. 2017; 12:e0187313. PMID: 29095918.
Article20. Nemes E, Rozot V, Geldenhuys H, Bilek N, Mabwe S, Abrahams D, et al. Optimization and interpretation of serial QuantiFERON testing to measure acquisition of Mycobacterium tuberculosis infection. Am J Respir Crit Care Med. 2017; 196:638–648. PMID: 28737960.21. Moses MW, Zwerling A, Cattamanchi A, Denkinger CM, Banaei N, Kik SV, et al. Serial testing for latent tuberculosis using QuantiFERON-TB Gold In-Tube: a Markov model. Sci Rep. 2016; 6:30781. PMID: 27469388.
Article22. Kang YA, Lee HW, Yoon HI, Cho B, Han SK, Shim YS, et al. Discrepancy between the tuberculin skin test and the whole-blood interferon gamma assay for the diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country. JAMA. 2005; 293:2756–2761. PMID: 15941805.23. Yoo JW, Jo KW, Park GY, Shim TS. Comparison of latent tuberculosis infection rate between contacts with active tuberculosis and non-contacts. Respir Med. 2016; 111:77–83. PMID: 26725461.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Usefulness of the Tuberculosis Skin Test and the Interferon-gamma Release Assay in the Diagnosis of Latent Tuberculosis Infection in South Korea
- Diagnosis and treatment of latent tuberculosis infection
- Prevalence and Risk Factors of Latent Tuberculosis Infection among Healthcare Workers Using Tuberculin Skin Test and Interferon-γ Release Assay at a Tertiary Hospital in South Korea
- Proposal to Revise the Screening Test for Latent Tuberculosis Infection in Close Contacts at Elementary Schools in Korea
- Usefulness of interferon-γ release assay for the diagnosis of latent tuberculosis infection in young children