Yonsei Med J.
2019 Apr;60(4):326-335. 10.3349/ymj.2019.60.4.326.
Potential Oncogenic Role of the Papillary Renal Cell Carcinoma Gene in Non-Small Cell Lung Cancers
- Affiliations
-
- 1Molecular Cell Biology, Department of Biomedicine & Health Sciences, The Catholic University of Korea, Seoul, Korea. yejun@catholic.ac.kr
- 2Precision Medicine Research Center, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Integrated Research Center for Genome Polymorphism, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 4Department of Microbiology, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 5Department of Immunology, Medicine & Pharmacy Research Center, Binzhou Medical University, Yantai, China.
- 6Cancer Evolution Research Center, The Catholic University of Korea, Seoul, Korea.
- 7Department of Hospital Pathology, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2450187
- DOI: http://doi.org/10.3349/ymj.2019.60.4.326
Abstract
- PURPOSE
Papillary renal cell carcinoma (PRCC) gene, which located in 1q23.1, is recurrently amplified in non-small cell lung cancer (NSCLC). However, it is unknown whether PRCC is overexpressed in primary NSCLCs and whether PRCC overexpression contributes to lung tumorigenesis. In this study, we aimed to identify the profiles of PRCC expression in Korean NSCLC patients and to elucidate the role of PRCC overexpression on lung tumorigenesis.
MATERIALS AND METHODS
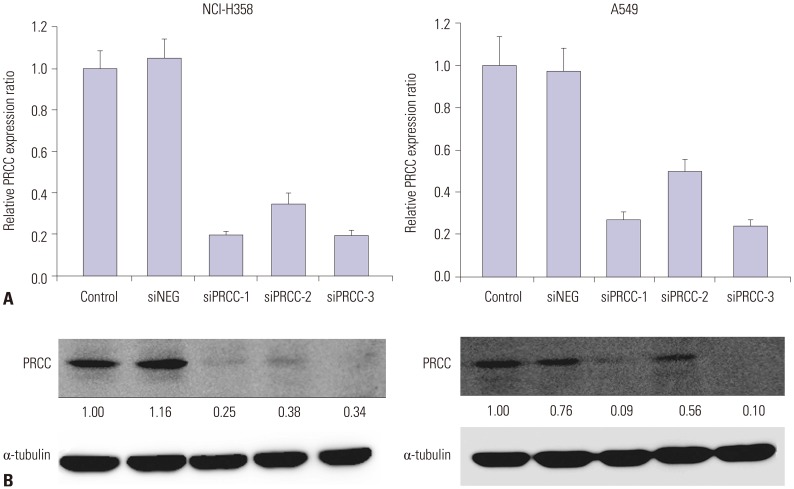
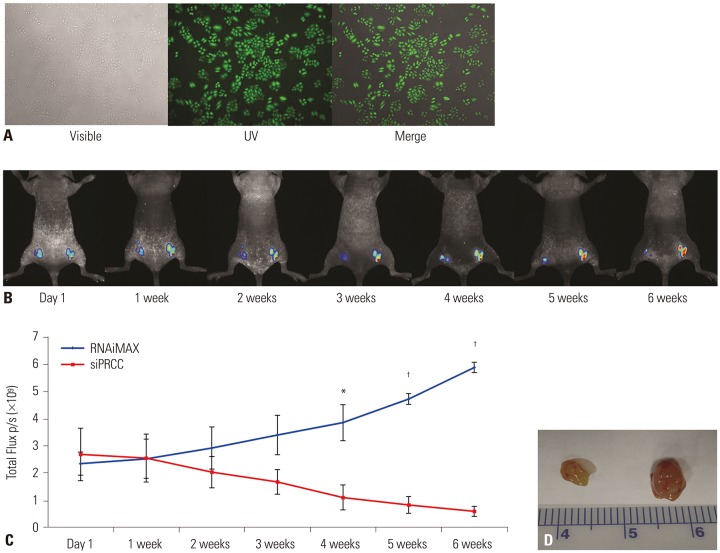
We performed immunohistochemistry analysis with a tissue array containing 161 primary NSCLCs. Small interfering RNA targeting PRCC (siPRCC) was transfected into two lung cancer cell lines (NCI-H358 and A549), after which tumor growth, migration, and invasion were observed. Expressions of cell proliferation-, cell cycle-, and metastasis-related molecules were examined by Western blot analysis. We also explored the in vivo effect of PRCC silencing.
RESULTS
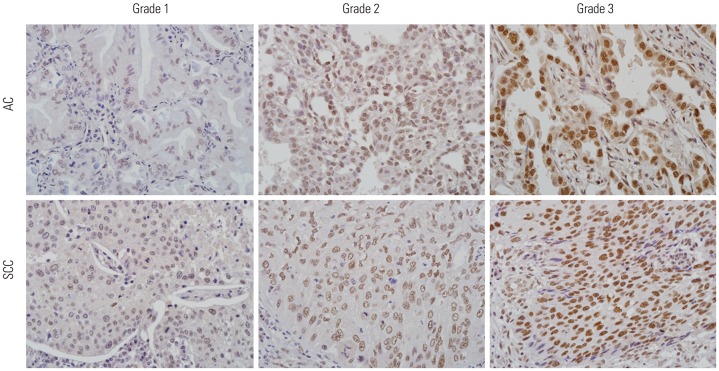
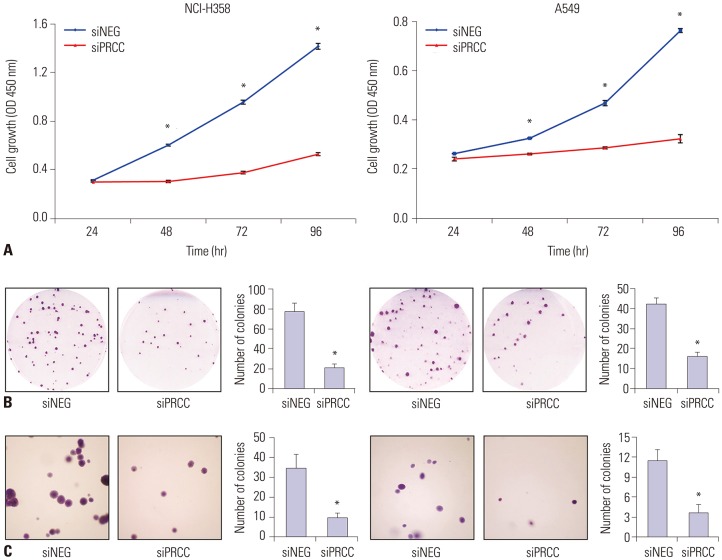
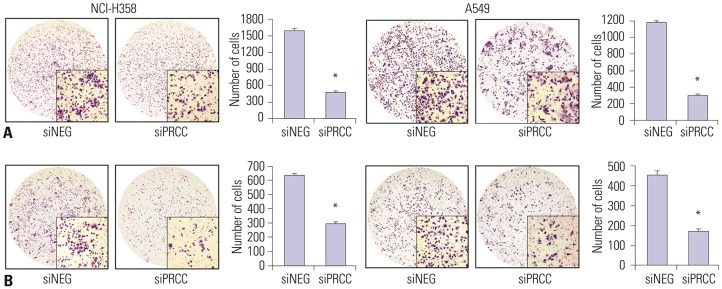
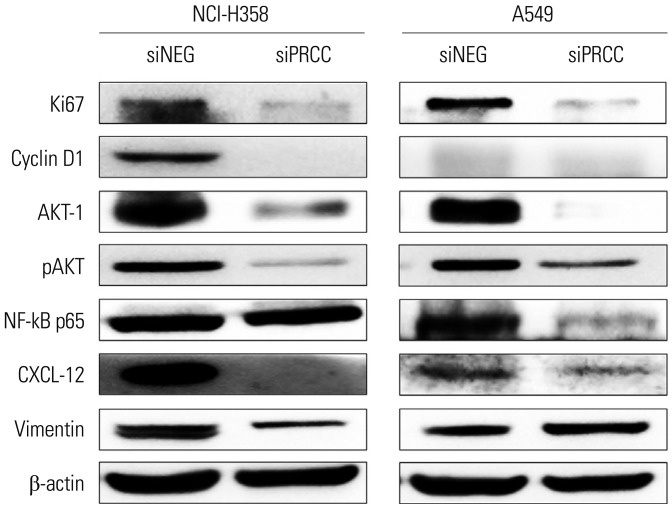
PRCC overexpression was recurrently observed in NSCLCs (95/161, 59%). After siPRCC treatment, tumor cell proliferation, colony formation, and anchorage independent growth were significantly reduced (p < 0.001 for all three effects). Migration and invasiveness were also significantly repressed (p < 0.001 for both effects). Reflecting cell proliferation, cell cycle, and metastasis, the expressions of Ki67, cyclin D1, AKT-1, pAKT, NF-kB p65, vimentin and CXCL-12 were found to be downregulated. Through mouse xenograft analysis, we confirmed that PRCC silencing significantly repressed a xenograft tumor mass in vivo (p < 0.001).
CONCLUSION
The present data provide evidence that PRCC overexpression is involved in the tumorigenesis and progression of lung cancer.
Keyword
MeSH Terms
-
Animals
Blotting, Western
Carcinogenesis
Carcinoma, Non-Small-Cell Lung
Carcinoma, Renal Cell*
Cell Cycle
Cell Line
Cell Proliferation
Cyclin D1
Heterografts
Humans
Immunohistochemistry
Lung Neoplasms*
Lung*
Mice
Neoplasm Metastasis
NF-kappa B
RNA, Small Interfering
Vimentin
Cyclin D1
NF-kappa B
RNA, Small Interfering
Vimentin
Figure
Reference
-
1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010; 60:277–300. PMID: 20610543.
Article2. Cafarotti S, Lococo F, Froesh P, Zappa F, Andrè D. Target therapy in lung cancer. Adv Exp Med Biol. 2016; 893:127–136. PMID: 26667341.
Article3. VanderLaan PA, Rangachari D, Mockus SM, Spotlow V, Reddi HV, Malcolm J, et al. Mutations in TP53, PIK3CA, PTEN and other genes in EGFR mutated lung cancers: correlation with clinical outcomes. Lung Cancer. 2017; 106:17–21. PMID: 28285689.4. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009; 361:947–957. PMID: 19692680.
Article5. Ke EE, Wu YL. EGFR as a pharmacological target in EGFR-mutant non-small-cell lung cancer: where do we stand now? Trends Pharmacol Sci. 2016; 37:887–903. PMID: 27717507.6. Kim TM, Yim SH, Lee JS, Kwon MS, Ryu JW, Kang HM, et al. Genome-wide screening of genomic alterations and their clinicopathologic implications in non-small cell lung cancers. Clin Cancer Res. 2005; 11:8235–8242. PMID: 16322280.
Article7. Sidhar SK, Clark J, Gill S, Hamoudi R, Crew AJ, Gwilliam R, et al. The t(X;1)(p11.2;q21.2) translocation in papillary renal cell carcinoma fuses a novel gene PRCC to the TFE3 transcription factor gene. Hum Mol Genet. 1996; 5:1333–1338. PMID: 8872474.
Article8. Weterman MA, Wilbrink M, Geurts van Kessel A. Fusion of the transcription factor TFE3 gene to a novel gene, PRCC, in t(X;1) (p11;q21)-positive papillary renal cell carcinomas. Proc Natl Acad Sci U S A. 1996; 93:15294–15298. PMID: 8986805.9. Pérot C, Boccon-Gibod L, Bouvier R, Doz F, Fournet JC, Fréneaux P, et al. Five new cases of juvenile renal cell carcinoma with translocations involving Xp11.2: a cytogenetic and morphologic study. Cancer Genet Cytogenet. 2003; 143:93–99. PMID: 12781442.10. Weterman MA, Wilbrink M, Eleveld M, Merkx G, van Groningen JJ, van Rooijen M, et al. Genomic structure, chromosomal localization, and embryonic expression of the mouse homolog of PRCC, a gene associated with papillary renal cell carcinoma. Cytogenet Cell Genet. 2001; 92:326–332. PMID: 11435707.
Article11. Mayer BJ. SH3 domains: complexity in moderation. J Cell Sci. 2001; 114(Pt 7):1253–1263. PMID: 11256992.
Article12. Weterman MA, van Groningen JJ, Tertoolen L, van Kessel AG. Impairment of MAD2B-PRCC interaction in mitotic checkpoint defective t(X;1)-positive renal cell carcinomas. Proc Natl Acad Sci U S A. 2001; 98:13808–13813. PMID: 11717438.
Article13. van den Hurk WH, Martens GJ, Geurts van Kessel A, van Groningen JJ. Isolation and characterization of the Xenopus laevis orthologs of the human papillary renal cell carcinoma-associated genes PRCC and MAD2L2 (MAD2B). Cytogenet Genome Res. 2004; 106:68–73. PMID: 15218244.14. Liu J, Kim SY, Shin S, Jung SH, Yim SH, Lee JY, et al. Overexpression of TFF3 is involved in prostate carcinogenesis via blocking mitochondria-mediated apoptosis. Exp Mol Med. 2018; 50:110. PMID: 30139961.
Article15. Jiang Y, Yim SH, Xu HD, Jung SH, Yang SY, Hu HJ, et al. A potential oncogenic role of the commonly observed E2F5 overexpression in hepatocellular carcinoma. World J Gastroenterol. 2011; 17:470–477. PMID: 21274376.
Article16. Xu H, Choe C, Shin SH, Park SW, Kim HS, Jung SH, et al. Silencing of KIF14 interferes with cell cycle progression and cytokinesis by blocking the p27(Kip1) ubiquitination pathway in hepatocellular carcinoma. Exp Mol Med. 2014; 46:e97. PMID: 24854087.
Article17. Vissers LE, Veltman JA, van Kessel AG, Brunner HG. Identification of disease genes by whole genome CGH arrays. Hum Mol Genet. 2005; 14 Spec No. 2:R215–R223. PMID: 16244320.
Article18. Kim TM, Yim SH, Shin SH, Xu HD, Jung YC, Park CK, et al. Clinical implication of recurrent copy number alterations in hepatocellular carcinoma and putative oncogenes in recurrent gains on 1q. Int J Cancer. 2008; 123:2808–2815. PMID: 18803288.
Article19. Tai AL, Yan WS, Fang Y, Xie D, Sham JS, Guan XY. Recurrent chromosomal imbalances in nonsmall cell lung carcinoma: the association between 1q amplification and tumor recurrence. Cancer. 2004; 100:1918–1927. PMID: 15112273.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Papillary Type of Renal Cell Carcinoma after Renal Injury in a Child
- Fine Needle Aspiration Cytology of Papillary Renal Cell Carcinoma: A Case Report
- Two Cases of Non-Small Cell Lung Cancer Masquerading as Metastatic Papillary Thyroid Cancer
- Multiple primary lung cancer: Synchronous small cell lung carcinoma and squamous cell carcinoma
- Mutational Analysis of PUMA Gene in Non-small Cell Lung Cancers