J Breast Cancer.
2019 Jun;22(2):274-284. 10.4048/jbc.2019.22.e28.
Murine Model Study of a New Receptor-Targeted Tracer for Sentinel Lymph Node in Breast Cancer
- Affiliations
-
- 1School of Medicine and Life Sciences, University of Jinan and Shandong Academy of Medical Sciences, Jinan, China.
- 2Breast Cancer Center, Shandong Cancer Hospital affiliated to Shandong University, Shandong Academy of Medical Sciences, Jinan, China. wangysh2008@aliyun.com
- KMID: 2450121
- DOI: http://doi.org/10.4048/jbc.2019.22.e28
Abstract
- PURPOSE
Sentinel lymph node biopsy (SLNB), a critical staging and treatment step, has replaced axillary lymph node (LN) dissection as the standard staging procedure for early stage breast cancer patients with clinically negative axillary LNs. Hence, using a murine sentinel lymph node (SLN) model, we investigated the localization effect of the new receptor-targeted tracer, indocyanine green (ICG)-rituximab, on breast cancer SLNB.
METHODS
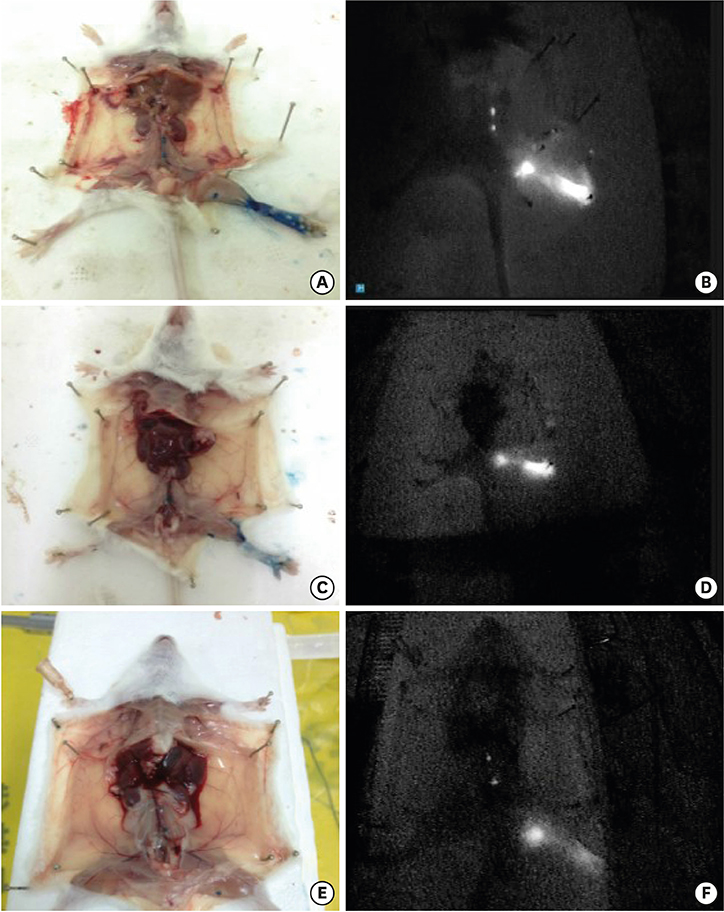
After establishing the murine SLN model, different doses of ICG-rituximab were subcutaneously injected into the hind insteps of BALB/c mice to determine the optimal dose and imaging time using continuous (> 3 hours) MDM-I fluorescence vasculature imaging. To explore the capacity of ICG-rituximab for sustained SLN localization with the optimal dose, MDM-I imaging was monitored at 6, 12, and 24 hours.
RESULTS
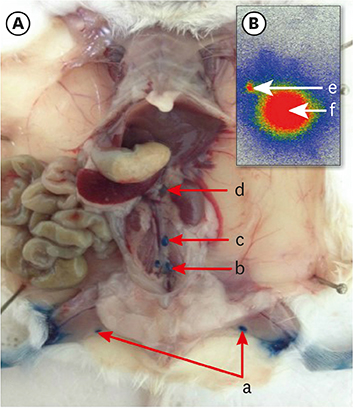
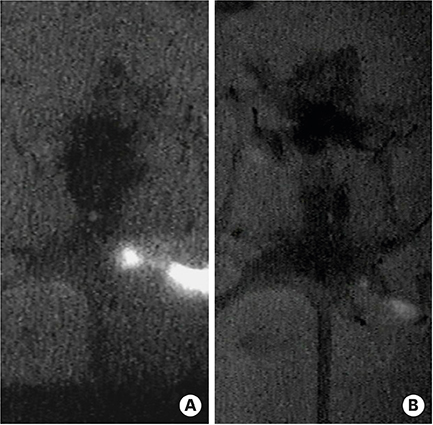
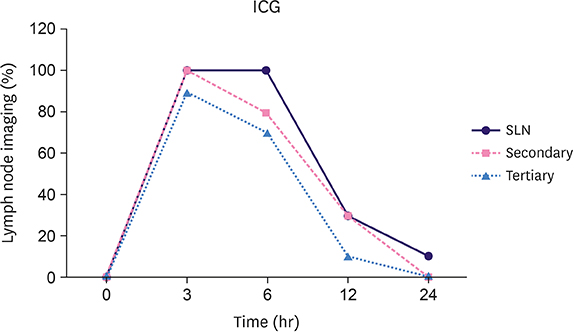
The popliteal LN was defined as the SLN for hindlimb lymphatic drainage, the iliac LN as the secondary, and the para-aortic or renal LN as the tertiary LNs. The SLN initial imaging and optimal imaging times were shortened with increased ICG-rituximab doses, and the imaging rates of the secondary and tertiary LNs increased accordingly. The optimal ICG dose was 0.12 μg, and its optimal imaging time was 34 minutes. After 24 hours, the SLN imaging rate remained 100%, while those of the secondary and the tertiary LNs increased from 0% (6 hours) and 0% (6 hours) to 10% (12 hours) and 10% (12 hours) to 20% (24 hours) and 10% (24 hours), respectively.
CONCLUSION
ICG-rituximab localized to the SLN without imaging from the secondary or tertiary LNs within 6 hours. The optimal ICG dose was 0.12 μg, and the optimal interval for SLN detection was 34 minutes to 6 hours post-injection. This novel receptor-targeted tracer is of great value to clinical research and application.
MeSH Terms
Figure
Reference
-
1. Lyman GH, Temin S, Edge SB, Newman LA, Turner RR, Weaver DL, et al. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2014; 32:1365–1383.
Article2. Veronesi U, Paganelli G, Galimberti V, Viale G, Zurrida S, Bedoni M, et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet. 1997; 349:1864–1867.
Article3. Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Ashikaga T, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007; 8:881–888.
Article4. Zavagno G, De Salvo GL, Scalco G, Bozza F, Barutta L, Del Bianco P, et al. A randomized clinical trial on sentinel lymph node biopsy versus axillary lymph node dissection in breast cancer: results of the Sentinella/GIVOM trial. Ann Surg. 2008; 247:207–213.
Article5. Hirche C, Murawa D, Mohr Z, Kneif S, Hünerbein M. ICG fluorescence-guided sentinel node biopsy for axillary nodal staging in breast cancer. Breast Cancer Res Treat. 2010; 121:373–378.
Article6. Stratmann SL, McCarty TM, Kuhn JA. Radiation safety with breast sentinel node biopsy. Am J Surg. 1999; 178:454–457.
Article7. Wishart GC, Loh SW, Jones L, Benson JR. A feasibility study (ICG-10) of indocyanine green (ICG) fluorescence mapping for sentinel lymph node detection in early breast cancer. Eur J Surg Oncol. 2012; 38:651–656.
Article8. Chi C, Ye J, Ding H, He D, Huang W, Zhang GJ, et al. Use of indocyanine green for detecting the sentinel lymph node in breast cancer patients: from preclinical evaluation to clinical validation. PLoS One. 2013; 8:e83927.
Article9. Vahrmeijer AL, Hutteman M, van der Vorst JR, van de Velde CJ, Frangioni JV. Image-guided cancer surgery using near-infrared fluorescence. Nat Rev Clin Oncol. 2013; 10:507–518.
Article10. Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994; 83:435–445.
Article11. Van den Broeck W, Derore A, Simoens P. Anatomy and nomenclature of murine lymph nodes: descriptive study and nomenclatory standardization in BALB/cAnNCrl mice. J Immunol Methods. 2006; 312:12–19.
Article12. Turner RR, Ollila DW, Krasne DL, Giuliano AE. Histopathologic validation of the sentinel lymph node hypothesis for breast carcinoma. Ann Surg. 1997; 226:271–278.
Article13. Krag D, Weaver D, Ashikaga T, Moffat F, Klimberg VS, Shriver C, et al. The sentinel node in breast cancer--a multicenter validation study. N Engl J Med. 1998; 339:941–946.
Article14. Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003; 349:546–553.
Article15. Degnim AC, Oh K, Cimmino VM, Diehl KM, Chang AE, Newman LA, et al. Is blue dye indicated for sentinel lymph node biopsy in breast cancer patients with a positive lymphoscintigram? Ann Surg Oncol. 2005; 12:712–717.
Article16. Ahmed M, Purushotham AD, Douek M. Novel techniques for sentinel lymph node biopsy in breast cancer: a systematic review. Lancet Oncol. 2014; 15:e351–e362.
Article17. Kelley LM, Holmes DR. Tracer agents for the detection of sentinel lymph nodes in breast cancer: current concerns and directions for the future. J Surg Oncol. 2011; 104:91–96.
Article18. Guo W, Zhang L, Ji J, Gao W, Liu J, Tong M. Breast cancer sentinel lymph node mapping using near-infrared guided indocyanine green in comparison with blue dye. Tumour Biol. 2014; 35:3073–3078.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Number of Removed Lymph Nodes for an Acceptable False Negative Rate in Sentinel Lymph Node Biopsy for Breast Cancer
- Validation and Controversy of Sentinel Node Biopsy for Breast Cancer
- Sentinel Lymph Node Imaging in Breast Cancer
- Sentinel Lymph Node Biopsy in Breast Cancer: A Clinical Review and Update
- Endoscopic Sentinel Lymph Node Biopsy in Breast Cancer Surgery: Feasibility and Accuracy of the Combined Radioisotope and Blue Dye