J Breast Cancer.
2019 Jun;22(2):172-184. 10.4048/jbc.2019.22.e21.
Overexpression of Tumor Protein p53-regulated Apoptosis-inducing Protein 1 Regulates Proliferation and Apoptosis of Breast Cancer Cells through the PI3K/Akt Pathway
- Affiliations
-
- 1Department of Breast Surgery, The Third Affiliated Hospital of Chongqing Medical University, Chongqing, China.
- 2Department of Breast & Thyroid Surgery, Southwest Hospital, Third Military Medical University(Army Medical University), Chongqing, China.
- 3Department of Breast & Thyroid Surgery, The First People's Hospital of Yunnan Province, Kunming, China. jialiu_lj12@163.com
- KMID: 2450113
- DOI: http://doi.org/10.4048/jbc.2019.22.e21
Abstract
- PURPOSE
Tumor protein p53-regulated apoptosis-inducing protein 1 (TP53AIP1) functions in various cancers. We studied the effect and molecular mechanism of TP53AIP1 in breast cancer.
METHODS
The degree of correlation between TP53AIP1 expression and overall survival in patients with breast cancer was obtained from the online The Cancer Genome Atlas database. Six of the TP53AIP1 levels in the tumor and adjacent non-tumor tissues randomly selected from 38 breast cancer patients were determined. Transgenic technology was used to enhance the expression of TP53AIP1 in breast cancer cell lines, MDA-MB-415 and MDA-MB-468, and to observe the effects of gene overexpression on the proliferation, cell cycle, and apoptosis of breast cancer cells. The molecular mechanism of association between cell cycle- and apoptosis-related factors and the phosphoinositide 3-kinases/protein kinase B (PI3K/Akt) pathway was also studied.
RESULTS
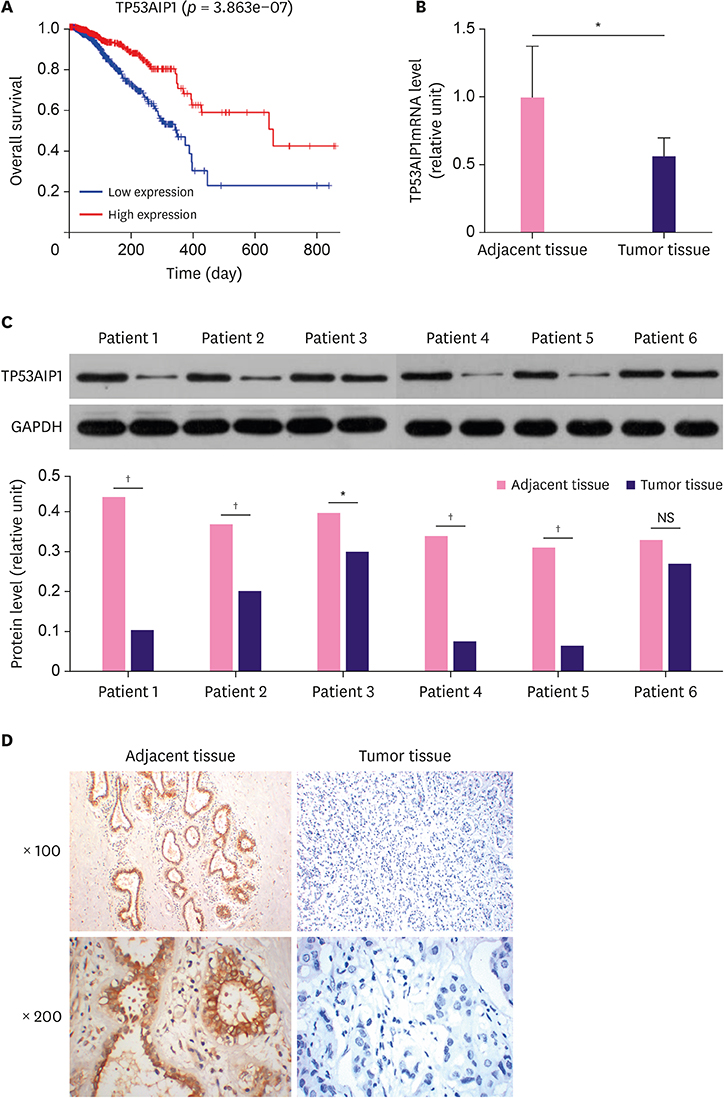
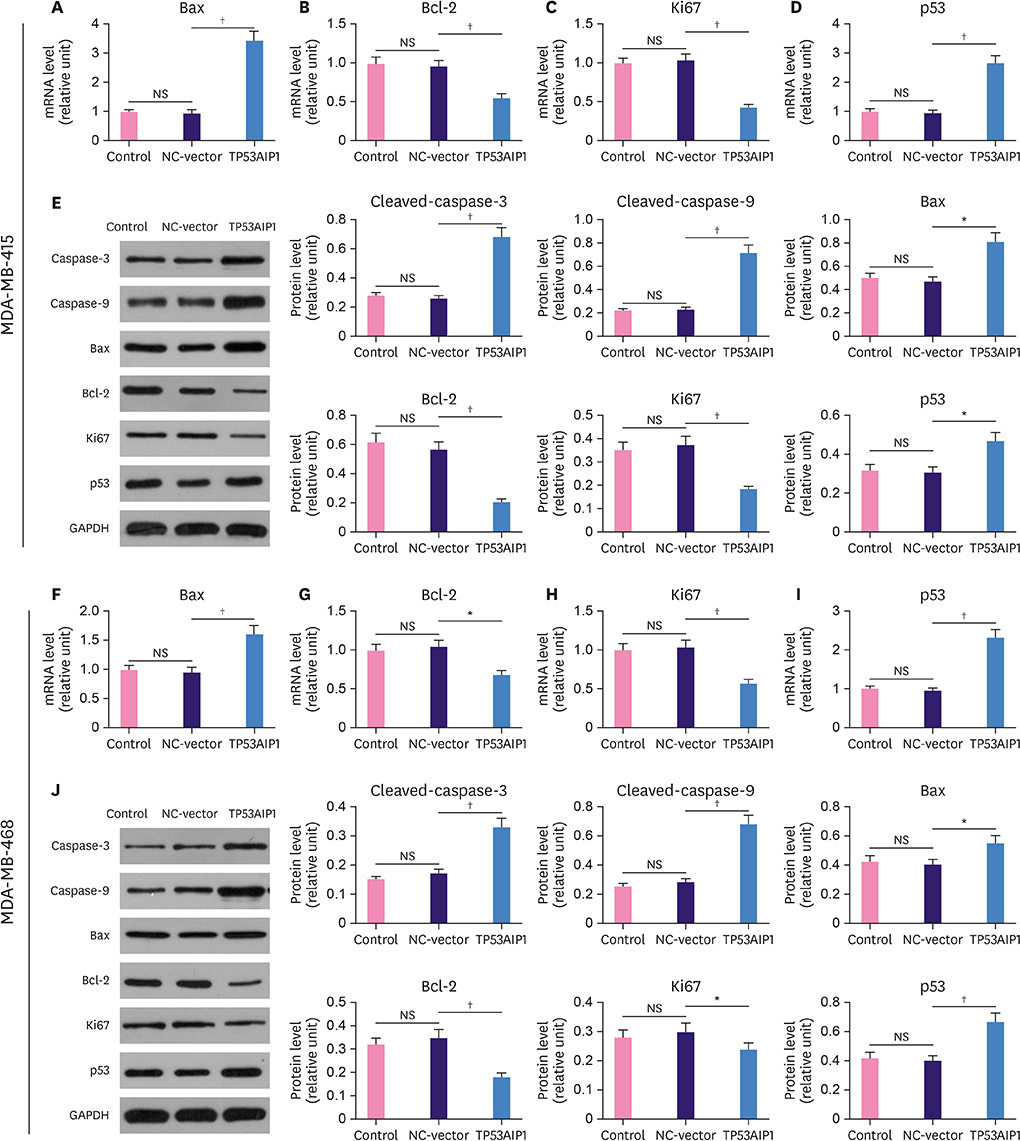
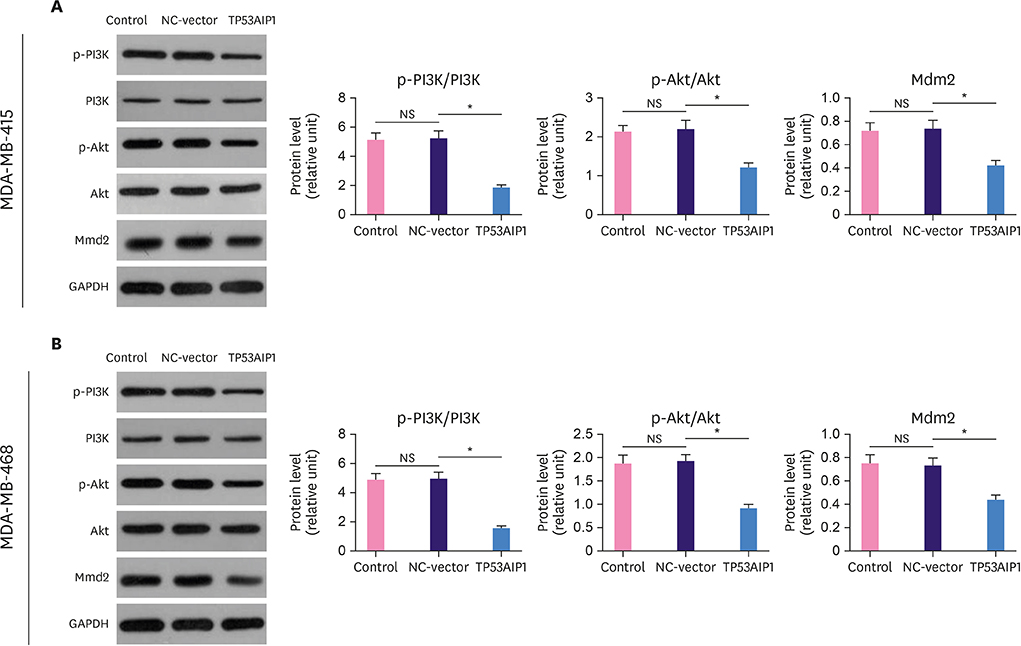
The messenger RNA and protein expression levels of TP53AIP1 in cancer tissues were significantly lower than those in the control group. TP53AIP1 overexpression inhibits cell viability. The mechanism of TP53AIP1 inhibition of proliferation and growth of breast cancer cells includes cell cycle arrest, apoptosis promotion (p < 0.01), promotion of the expression of cleaved-caspase-3 (p < 0.01), cleaved-caspase-9 (p < 0.01), B cell lymphoma/leukemia-2 (Bcl-2)-associated X protein, and p53 (p < 0.01), and the inhibition of Bcl-2, Ki67, and PI3K/Akt pathways (p < 0.01).
CONCLUSION
TP53AIP1 may be a novel tumor suppressor gene in breast cancer and can potentially be used as an effective target gene for the treatment of breast cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Luedeke M, Coinac I, Linnert CM, Bogdanova N, Rinckleb AE, Schrader M, et al. Prostate cancer risk is not altered by TP53AIP1 germline mutations in a German case-control series. PLoS One. 2012; 7:e34128.
Article2. Matsuda K, Yoshida K, Taya Y, Nakamura K, Nakamura Y, Arakawa H. p53AIP1 regulates the mitochondrial apoptotic pathway. Cancer Res. 2002; 62:2883–2889.3. Oda K, Arakawa H, Tanaka T, Matsuda K, Tanikawa C, Mori T, et al. p53AIP1, a potential mediator of p53-dependent apoptosis, and its regulation by Ser-46-phosphorylated p53. Cell. 2000; 102:849–862.
Article4. Jiang Y, Chen H, Jia H, Xu Y, Liu G, Wang Y, et al. Adenovirus Ad-p53AIP1-mediated gene therapy and its regulation of p53-MDM2 interactions. Exp Ther Med. 2010; 1:363–368.
Article5. Yamashita SI, Masuda Y, Yoshida N, Matsuzaki H, Kurizaki T, Haga Y, et al. p53AIP1 expression can be a prognostic marker in non-small cell lung cancer. Clin Oncol (R Coll Radiol). 2008; 20:148–151.
Article6. Hillberg NS, Meesters-Caberg MA, Beugels J, Winkens B, Vissers YL, van Mulken TJ. Delay of adjuvant radiotherapy due to postoperative complications after oncoplastic breast conserving surgery. Breast. 2018; 39:110–116.
Article7. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009; 59:225–249.
Article8. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013; 63:11–30.
Article9. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016; 66:7–30.
Article10. Özkurt E. Surgical highlights from the 40th San Antonio Breast Cancer Symposium: 5–9 December 2017, San Antonio, Texas. Eur J Breast Health. 2018; 14:74–79.
Article11. Denduluri N, Chavez-MacGregor M, Telli ML, Eisen A, Graff SL, Hassett MJ, et al. Selection of optimal adjuvant chemotherapy and targeted therapy for early breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol. 2018; 36:2433–2443.
Article12. Lin YS, Lin YY, Yang YH, Lin CL, Kuan FC, Lu CN, et al. Antrodia cinnamomea extract inhibits the proliferation of tamoxifen-resistant breast cancer cells through apoptosis and skp2/microRNAs pathway. BMC Complement Altern Med. 2018; 18:152.
Article13. Ma L, Liang Z, Zhou H, Qu L. Applications of RNA indexes for precision oncology in breast cancer. Genomics Proteomics Bioinformatics. 2018; 16:108–119.
Article14. Khan MI, Sobocińska AA, Brodaczewska KK, Zielniok K, Gajewska M, Kieda C, et al. Involvement of the CB2 cannabinoid receptor in cell growth inhibition and G0/G1 cell cycle arrest via the cannabinoid agonist WIN 55,212-2 in renal cell carcinoma. BMC Cancer. 2018; 18:583.
Article15. Muralidhar SA, Sadek HA. Meis1 regulates postnatal cardiomyocyte cell cycle arrest. In : Nakanishi T, Markwald RR, Baldwin HS, Keller BB, Srivastava D, Yamagishi H, editors. Etiology and Morphogenesis of Congenital Heart Disease: From Gene Function and Cellular Interaction to Morphology. Tokyo: Springer Japan;2016. p. 93–101.16. Qiao L, Zheng J, Tian Y, Zhang Q, Wang X, Chen JJ, et al. Regulator of chromatin condensation 1 abrogates the G1 cell cycle checkpoint via Cdk1 in human papillomavirus E7-expressing epithelium and cervical cancer cells. Cell Death Dis. 2018; 9:583.
Article17. Wang Y, Wang R, Li Y, Sun Y, Song C, Zhan Y, et al. Newcastle disease virus induces G0/G1 cell cycle arrest in asynchronously growing cells. Virology. 2018; 520:67–74.
Article18. Jin CY, Molagoda IMN, Karunarathne WAHM, Kang SH, Park C, Kim GY, et al. TRAIL attenuates sulforaphane-mediated Nrf2 and sustains ROS generation, leading to apoptosis of TRAIL-resistant human bladder cancer cells. Toxicol Appl Pharmacol. 2018; 352:132–141.
Article19. Le PM, Silvestri VL, Redstone SC, Dunn JB, Millard JT. Cross-linking by epichlorohydrin and diepoxybutane correlates with cytotoxicity and leads to apoptosis in human leukemia (HL-60) cells. Toxicol Appl Pharmacol. 2018; 352:19–27.
Article20. Li Y, Pan G, Chen Y, Yang Q, Hao T, Zhao L, et al. Inhibitor of the human telomerase reverse trancriptase (hTERT) gene promoter induces cell apoptosis via a mitochondrial-dependent pathway. Eur J Med Chem. 2018; 145:370–378.
Article21. Yu Y, Wu X, Pu J, Luo P, Ma W, Wang J, et al. Lycium barbarum polysaccharide protects against oxygen glucose deprivation/reoxygenation-induced apoptosis and autophagic cell death via the PI3K/Akt/mTOR signaling pathway in primary cultured hippocampal neurons. Biochem Biophys Res Commun. 2018; 495:1187–1194.
Article22. Suliman FA, Khodeer DM, Ibrahiem A, Mehanna ET, El-Kherbetawy MK, Mohammad HM, et al. Renoprotective effect of the isoflavonoid biochanin A against cisplatin induced acute kidney injury in mice: effect on inflammatory burden and p53 apoptosis. Int Immunopharmacol. 2018; 61:8–19.
Article23. Lima KG, Krause GC, da Silva EF, Xavier LL, Martins LA, Alice LM, et al. Octyl gallate reduces ATP levels and Ki67 expression leading HepG2 cells to cell cycle arrest and mitochondria-mediated apoptosis. Toxicol In Vitro. 2018; 48:11–25.
Article24. Ren Z, Yang T, Ding J, Liu W, Meng X, Zhang P, et al. MiR-520d-3p antitumor activity in human breast cancer via post-transcriptional regulation of spindle and kinetochore associated 2 expression. Am J Transl Res. 2018; 10:1097–1108.25. Li H, Wang Y, Chen B, Shi J. Silencing of PAQR3 suppresses extracellular matrix accumulation in high glucose-stimulated human glomerular mesangial cells via PI3K/AKT signaling pathway. Eur J Pharmacol. 2018; 832:50–55.
Article26. Wu C, Jiang F, Wei K, Jiang Z.. Exercise activates the PI3K-AKT signal pathway by decreasing the expression of 5α-reductase type 1 in PCOS rats. Sci Rep. 2018; 8:7982.
Article27. Amaral C, Augusto TV, Tavares-da-Silva E, Roleira FM, Correia-da-Silva G, Teixeira N. Hormone-dependent breast cancer: targeting autophagy and PI3K overcomes exemestane-acquired resistance. J Steroid Biochem Mol Biol. 2018; 183:51–61.
Article28. Wei J, Wu J, Xu W, Nie H, Zhou R, Wang R, et al. Salvianolic acid B inhibits glycolysis in oral squamous cell carcinoma via targeting PI3K/AKT/HIF-1α signaling pathway. Cell Death Dis. 2018; 9:599.
Article29. Makise N, Sekimizu M, Kubo T, Wakai S, Watanabe SI, Kato T, et al. Extraskeletal osteosarcoma: MDM2 and H3K27me3 analysis of 19 cases suggest disease heterogeneity. Histopathology. 2018; 73:147–156.
Article30. Kim H, Ronai ZA. Rewired Notch/p53 by Numb'ing Mdm2. J Cell Biol. 2018; 217:445–446.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- LETM1 Promotes Gastric Cancer Cell Proliferation, Migration, and Invasion via the PI3K/Akt Signaling Pathway
- Gallic Acid Hindered Lung Cancer Progression by Inducing Cell Cycle Arrest and Apoptosis in A549 Lung Cancer Cells via PI3K/Akt Pathway
- PS-341-Induced Apoptosis is Related to JNK-Dependent Caspase 3 Activation and It is Negatively Regulated by PI3K/Akt-Mediated Inactivation of Glycogen Synthase Kinase-3beta in Lung Cancer Cells
- MSCs-Derived miR-150-5p-Expressing Exosomes Promote Skin Wound Healing by Activating PI3K/AKT Pathway through PTEN
- Dioscin Decreases Breast Cancer Stem-like Cell Proliferation via Cell Cycle Arrest by Modulating p38 Mitogen-activated Protein Kinase and AKT/mTOR Signaling Pathways