Intest Res.
2019 Apr;17(2):202-209. 10.5217/ir.2018.00086.
The novel latex agglutination turbidimetric immunoassay system for simultaneous measurements of calprotectin and hemoglobin in feces
- Affiliations
-
- 1Department of Gastroenterology and Hepatology, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan. sakikoh@cc.okayama-u.ac.jp
- 2Department of Endoscopy, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan.
- 3Department of Biochemical Research Laboratory-I, Eiken Chemical Co., Ltd., Tochigi, Japan.
- 4Department of Gastroenterology, Mitsui Memorial Hospital, Tokyo, Japan.
- KMID: 2449961
- DOI: http://doi.org/10.5217/ir.2018.00086
Abstract
- BACKGROUND/AIMS
Fecal calprotectin (Fcal) as well as the fecal immunochemical test (FIT) are useful biomarkers for detecting activity and mucosal healing in inflammatory bowel diseases. Here, we report the performance of simultaneous measurements of Fcal and FIT for ulcerative colitis (UC) patients using the newly-developed latex agglutination turbidimetric immunoassay (LATIA) system.
METHODS
Fcal and hemoglobin were measured by the LATIA system in 152 UC patients who underwent colonoscopy. Fcal was also quantified with a conventional enzyme-linked immunosorbent assay (ELISA). Fecal markers were evaluated in conjunction with the mucosal status of UC, which was assessed via the Mayo endoscopic subscore (MES) classification.
RESULTS
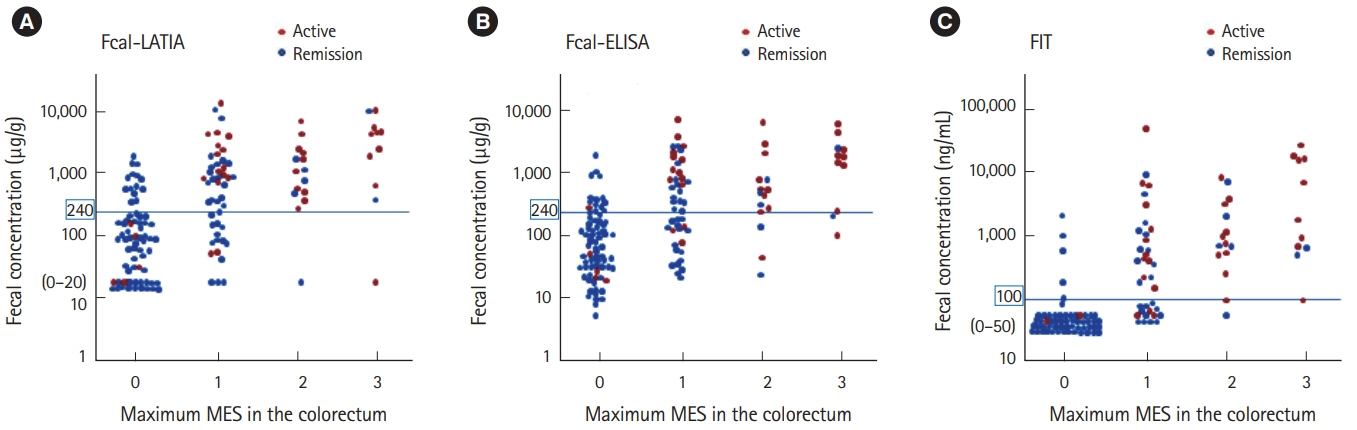
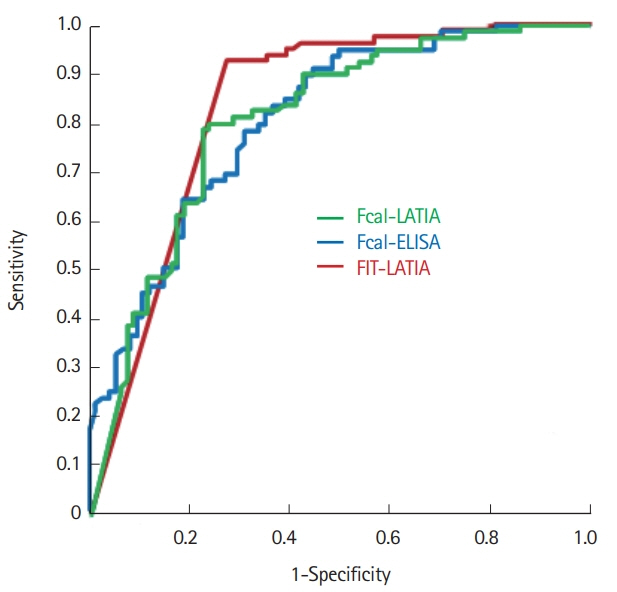
The LATIA system could quantify calprotectin and hemoglobin simultaneously with the same fecal samples within 10 minutes. The values of the Fcal-LATIA closely correlated with those of the Fcal-ELISA (Spearman rank correlation coefficient, r=0.84; P<0.0001). The values of Fcal for each assay and the FIT all significantly correlated with the MESs (Spearman rank correlation coefficient, Fcal-LATIA: r=0.58, Fcal-ELISA: r=0.55, and FIT: r=0.72). The mucosal healing predictability (determined by an MES of 0 alone) of the Fcal-LATIA, Fcal-ELISA, and FIT-LATIA with the cutoffs determined by receiver operating characteristic curve analysis was 0.79, 0.78, and 0.92 for sensitivity, respectively, and 0.78, 0.69, and 0.73 for specificity, respectively.
CONCLUSIONS
The performance of the novel Fcal-LATIA was equivalent to that of the conventional Fcal assay. Simultaneous measurements with FITs would promote the clinical relevance of fecal biomarkers in UC.
Keyword
MeSH Terms
Figure
Reference
-
1. Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002; 347:417–429.
Article2. Frøslie KF, Jahnsen J, Moum BA, Vatn MH; IBSEN Group. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007; 133:412–422.
Article3. Schoepfer AM, Beglinger C, Straumann A, Trummler M, Renzulli P, Seibold F. Ulcerative colitis: correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, C-reactive protein, and blood leukocytes. Inflamm Bowel Dis. 2009; 15:1851–1858.4. D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012; 18:2218–2224.
Article5. Takashima S, Kato J, Hiraoka S, et al. Evaluation of mucosal healing in ulcerative colitis by fecal calprotectin vs. fecal immunochemical test. Am J Gastroenterol. 2015; 110:873–880.
Article6. Hiraoka S, Kato J, Nakarai A, et al. Consecutive measurements by faecal immunochemical test in quiescent ulcerative colitis patients can detect clinical relapse. J Crohns Colitis. 2016; 10:687–694.
Article7. Hiraoka S, Inokuchi T, Nakarai A, et al. Fecal immunochemical test and fecal calprotectin results show different profiles in disease monitoring for ulcerative colitis. Gut Liver. 2018; 12:142–148.
Article8. Väänänen P, Tenhunen R. Rapid immunochemical detection of fecal occult blood by use of a latex-agglutination test. Clin Chem. 1988; 34:1763–1766.
Article9. Kusaka T, Nozaki T, Shibata M, et al. Basic measurement performance evaluation of fecal occult blood analyzer OC sensor PLEDIA. J Clin Lab Instru Reagents. 2014; 3:643–648.10. Wong WM, Lam SK, Cheung KL, et al. Evaluation of an automated immunochemical fecal occult blood test for colorectal neoplasia detection in a Chinese population. Cancer. 2003; 97:2420–2424.
Article11. Morikawa T, Kato J, Yamaji Y, Wada R, Mitsushima T, Shiratori Y. A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology. 2005; 129:422–428.
Article12. van der Vlugt M, Grobbee EJ, Bossuyt PM, et al. Interval colorectal cancer incidence among subjects undergoing multiple rounds of fecal immunochemical testing. Gastroenterology. 2017; 153:439–447. e2.
Article13. Vilkin A, Rozen P, Levi Z, et al. Performance characteristics and evaluation of an automated-developed and quantitative, immunochemical, fecal occult blood screening test. Am J Gastroenterol. 2005; 100:2519–2525.
Article14. Guittet L, Guillaume E, Levillain R, et al. Analytical comparison of three quantitative immunochemical fecal occult blood tests for colorectal cancer screening. Cancer Epidemiol Biomarkers Prev. 2011; 20:1492–1501.
Article15. Catomeris P, Baxter NN, Boss SC, et al. Effect of temperature and time on fecal hemoglobin stability in 5 fecal immunochemical test methods and one guaiac method. Arch Pathol Lab Med. 2018; 142:75–82.
Article16. Nakarai A, Kato J, Hiraoka S, et al. Evaluation of mucosal healing of ulcerative colitis by a quantitative fecal immunochemical test. Am J Gastroenterol. 2013; 108:83–89.
Article17. Nakarai A, Hiraoka S, Takahashi S, et al. Simultaneous measurements of faecal calprotectin and the faecal immunochemical test in quiescent ulcerative colitis patients can stratify risk of relapse. J Crohns Colitis. 2018; 12:71–76.
Article18. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis: a randomized study. N Engl J Med. 1987; 317:1625–1629.
Article19. Senju O, Takagi Y, Uzawa R, et al. A new immuno quantitative method by latex agglutination: application for the determination of serum C-reactive protein (CRP) and its clinical significance. J Clin Lab Immunol. 1986; 19:99–103.20. Martinuzzo ME, Ujhelly C, Barrera LH, et al. Validation of an automated immunoturbidimetric assay for fibrinogen/fibrin degradation products measurement and its correlation to a semi-quantitative latex agglutination test. Clin Lab. 2016; 62:2085–2089.
Article21. Westblom TU, Madan E, Gudipati S, Midkiff BR, Czinn SJ. Diagnosis of Helicobacter pylori infection in adult and pediatric patients by using Pyloriset, a rapid latex agglutination test. J Clin Microbiol. 1992; 30:96–98.
Article22. Mosli MH, Zou G, Garg SK, et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol. 2015; 110:802–819.
Article23. Nancey S, Boschetti G, Moussata D, et al. Neopterin is a novel reliable fecal marker as accurate as calprotectin for predicting endoscopic disease activity in patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2013; 19:1043–1052.
Article24. Ma C, Lumb R, Walker EV, et al. Noninvasive fecal immunochemical testing and fecal calprotectin predict mucosal healing in inflammatory bowel disease: a prospective cohort study. Inflamm Bowel Dis. 2017; 23:1643–1649.
Article25. Inoue K, Aomatsu T, Yoden A, Okuhira T, Kaji E, Tamai H. Usefulness of a novel and rapid assay system for fecal calprotectin in pediatric patients with inflammatory bowel diseases. J Gastroenterol Hepatol. 2014; 29:1406–1412.
Article26. Okuyama Y, Doi Y, Matsuyama N, Uchino M, Yamamoto T. A novel sol particle immunoassay for fecal calprotectin in inflammatory bowel disease patients. Clin Chim Acta. 2016; 456:1–6.
Article27. Labaere D, Smismans A, Van Olmen A, et al. Comparison of six different calprotectin assays for the assessment of inflammatory bowel disease. United European Gastroenterol J. 2014; 2:30–37.
Article28. Heida A, Knol M, Kobold AM, Bootsman J, Dijkstra G, van Rheenen PF. Agreement between home-based measurement of stool calprotectin and ELISA results for monitoring inflammatory bowel disease activity. Clin Gastroenterol Hepatol. 2017; 15:1742–1749. e2.
Article29. Bello C, Roseth A, Guardiola J, et al. Usability of a home-based test for the measurement of fecal calprotectin in asymptomatic IBD patients. Dig Liver Dis. 2017; 49:991–996.
Article30. Puolanne AM, Kolho KL, Alfthan H, Ristimäki A, Mustonen H, Färkkilä M. Rapid faecal tests for detecting disease activity in colonic inflammatory bowel disease. Eur J Clin Invest. 2016; 46:825–832.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Incidence and possible reasons for discordant results between positive FDP and negative D-dimer latex assays in clinical specimens
- Latex agglutination inhibition test(UCG-slide test) and monoconal antibody - based enzyme immunoassay test (RAMP test) in the diagnosis of ectopic pregnancy
- Evaluation of Enzyme Immunoassay for Urinary Pregnancy Tests
- Evaluation of Hitachi 7600-110(R) for Quantitative Analysis of C-Reactive Protein
- Latex Agglutination Test for Differentiating Neonatal Blood from the Maternal Blood in the Meconium