J Korean Neuropsychiatr Assoc.
2019 May;58(2):105-114. 10.4306/jknpa.2019.58.2.105.
Electroencephalography and Schizophrenia
- Affiliations
-
- 1Clinical Emotion and Cognition Research Laboratory, Inje University, Goyang, Korea. lshpss@hanmail.net
- 2Department of Psychiatry, Inje University Ilsan-Paik Hospital, Goyang, Korea.
- KMID: 2449053
- DOI: http://doi.org/10.4306/jknpa.2019.58.2.105
Abstract
- Electroencephalography (EEG) and event-related potentials (ERPs) are useful measures of information processing that are believed to reflect the cognitive processing of the brain. Recently, these electrophysiological markers have been studied repeatedly to examine patients with schizophrenia. Among the ERPs components, P50, P300, mismatch negativity, loudness dependence of auditory evoked potentials, and 40 Hz auditory steady state response are meaningful neurophysiological markers of schizophrenia. The employment of novel ERP paradigms designed to carefully characterize the early spectrum of perceptual and cognitive information processing allows investigators to identify the neurophysiological basis of cognitive dysfunction in schizophrenia and examine the associated clinical and functional impairments. Lately, functional neural networks using resting state EEG have been studied extensively in patients with schizophrenia. In this article, qEEG, several ERP components, and functional neural networks that were considered useful neurophysiological markers of schizophrenia are reviewed and their clinical implications are discussed.
Keyword
MeSH Terms
Figure
Reference
-
1. Hughes JR, John ER. Conventional and quantitative electroencephalography in psychiatry. J Neuropsychiatry Clin Neurosci. 1999; 11:190–208.
Article2. Yeum TS, Kang UG. Reduction in alpha peak frequency and coherence on quantitative electroencephalography in patients with schizophrenia. J Korean Med Sci. 2018; 33:e179.
Article3. Leiser SC, Dunlop J, Bowlby MR, Devilbiss DM. Review: aligning strategies for using EEG as a surrogate biomarker: a review of preclinical and clinical research. Biochem Pharmacol. 2011; 81:1408–1421.
Article4. Boutros NN, Arfken C, Galderisi S, Warrick J, Pratt G. The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophr Res. 2008; 99:225–237.
Article5. Gambini O, Colombo C, Macciardi F, Locatelli M, Calabrese G, Sacchetti E, et al. EEG power spectrum profile and structural CNS characteristics in schizophrenia. Biol Psychiatry. 1990; 27:1331–1334.
Article6. Sponheim SR, Clementz BA, Iacono WG, Beiser M. Resting EEG in first-episode and chronic schizophrenia. Psychophysiology. 1994; 31:37–43.
Article7. Omori M, Koshino Y, Murata T, Murata I, Nishio M, Sakamoto K, et al. Quantitative EEG in never-treated schizophrenic patients. Biol Psychiatry. 1995; 38:305–309.
Article8. Jetha MK, Schmidt LA, Goldberg JO. Resting frontal EEG asymmetry and shyness and sociability in schizophrenia: a pilot study of community-based outpatients. Int J Neurosci. 2009; 119:847–856.
Article9. Begić D, Hotujac L, Jokić-Begić N. Quantitative EEG in ‘positive’ and ‘negative’ schizophrenia. Acta Psychiatr Scand. 2000; 101:307–311.
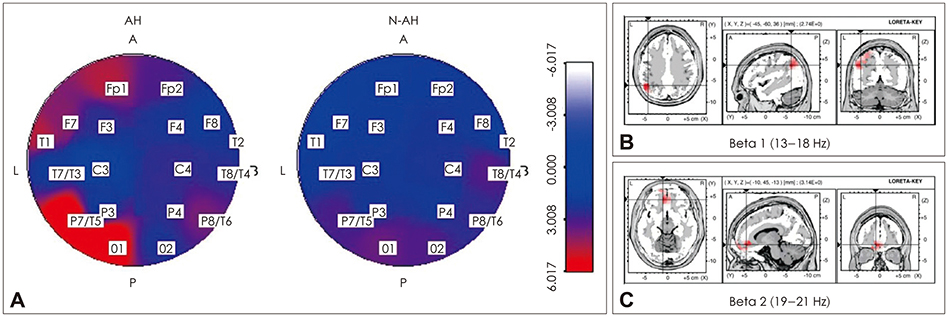
Article10. Lee SH, Wynn JK, Green MF, Kim H, Lee KJ, Nam M, et al. Quantitative EEG and low resolution electromagnetic tomography (LORETA) imaging of patients with persistent auditory hallucinations. Schizophr Res. 2006; 83:111–119.
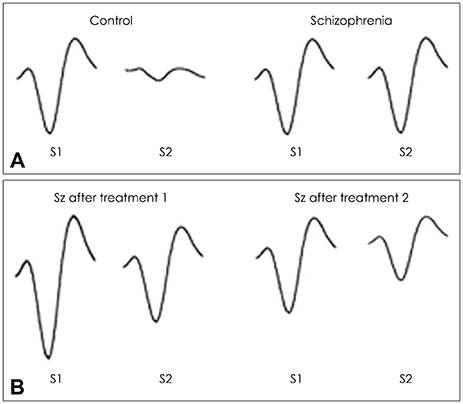
Article11. Adler LE, Pachtman E, Franks RD, Pecevich M, Waldo MC, Freedman R. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry. 1982; 17:639–654.12. McGhie A. Disorders of attention and perception in early schizophrenia. Br J Med Psychol. 1961; 34:103–116.
Article13. Sánchez-Morla EM, García-Jiménez MA, Barabash A, Martínez-Vizcaíno V, Mena J, Cabranes-Díaz JA, et al. P50 sensory gating deficit is a common marker of vulnerability to bipolar disorder and schizophrenia. Acta Psychiatr Scand. 2008; 117:313–318.
Article14. Vlcek P, Bob P, Raboch J. Sensory disturbances, inhibitory deficits, and the P50 wave in schizophrenia. Neuropsychiatr Dis Treat. 2014; 10:1309–1315.15. Freedman R, Adler LE, Waldo MC, Pachtman E, Franks RD. Neurophysiological evidence for a defect in inhibitory pathways in schizophrenia: comparison of medicated and drug-free patients. Biol Psychiatry. 1983; 18:537–551.16. Freedman R, Adler LE, Gerhardt GA, Waldo M, Baker N, Rose GM, et al. Neurobiological studies of sensory gating in schizophrenia. Schizophr Bull. 1987; 13:669–678.
Article17. Nagamoto HT, Adler LE, Waldo MC, Freedman R. Sensory gating in schizophrenics and normal controls: effects of changing stimulation interval. Biol Psychiatry. 1989; 25:549–561.
Article18. Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry. 1992; 49:206–215.
Article19. Brockhaus-Dumke A, Schultze-Lutter F, Mueller R, Tendolkar I, Bechdolf A, Pukrop R, et al. Sensory gating in schizophrenia: P50 and N100 gating in antipsychotic-free subjects at risk, first-episode, and chronic patients. Biol Psychiatry. 2008; 64:376–384.
Article20. Yee CM, Williams TJ, White PM, Nuechterlein KH, Ames D, Subotnik KL. Attentional modulation of the P50 suppression deficit in recent-onset and chronic schizophrenia. J Abnorm Psychol. 2010; 119:31–39.
Article21. Smucny J, Stevens KE, Olincy A, Tregellas JR. Translational utility of rodent hippocampal auditory gating in schizophrenia research: a review and evaluation. Transl Psychiatry. 2015; 5:e587.
Article22. Friedman D, Squires-Wheeler E. Event-related potentials (ERPs) as indicators of risk for schizophrenia. Schizophr Bull. 1994; 20:63–74.
Article23. Ford JM, Mathalon DH, Marsh L, Faustman WO, Harris D, Hoff AL, et al. P300 amplitude is related to clinical state in severely and moderately ill patients with schizophrenia. Biol Psychiatry. 1999; 46:94–101.
Article24. Bramon E, Rabe-Hesketh S, Sham P, Murray RM, Frangou S. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophr Res. 2004; 70:315–329.
Article25. Verleger R. On the utility of P3 latency as an index of mental chronometry. Psychophysiology. 1997; 34:131–156.
Article26. Goodin DS, Squires KC, Henderson BH, Starr A. Age-related variations in evoked potentials to auditory stimuli in normal human subjects. Electroencephalogr Clin Neurophysiol. 1978; 44:447–458.
Article27. Polich J. Meta-analysis of P300 normative aging studies. Psychophysiology. 1996; 33:334–353.
Article28. O'Donnell BF, McCarley RW, Potts GF, Salisbury DF, Nestor PG, Hirayasu Y, et al. Identification of neural circuits underlying P300 abnormalities in schizophrenia. Psychophysiology. 1999; 36:388–398.29. Wang J, Hirayasu Y, Hiramatsu K, Hokama H, Miyazato H, Ogura C. Increased rate of P300 latency prolongation with age in drug-naive and first episode schizophrenia. Clin Neurophysiol. 2003; 114:2029–2035.
Article30. Lim Y, Lee S, Hong S. Application of N100, P300 and QEEG as a biological marker in patients with schizophrenia. Korean Journal of Psychopharmacology. 2010; 21:78–86.31. Kim DW, Shim M, Kim JI, Im CH, Lee SH. Source activation of P300 correlates with negative symptom severity in patients with schizophrenia. Brain Topogr. 2014; 27:307–317.
Article32. Mathalon DH, Ford JM, Pfefferbaum A. Trait and state aspects of P300 amplitude reduction in schizophrenia: a retrospective longitudinal study. Biol Psychiatry. 2000; 47:434–449.
Article33. Bramon E, Shaikh M, Broome M, Lappin J, Bergé D, Day F, et al. Abnormal P300 in people with high risk of developing psychosis. Neuroimage. 2008; 41:553–560.
Article34. Ozgürdal S, Gudlowski Y, Witthaus H, Kawohl W, Uhl I, Hauser M, et al. Reduction of auditory event-related P300 amplitude in subjects with at-risk mental state for schizophrenia. Schizophr Res. 2008; 105:272–278.
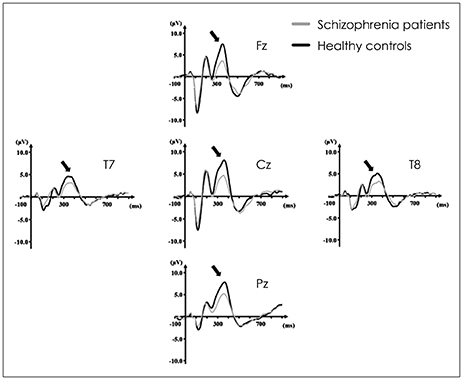
Article35. Näätänen R. Selective attention and evoked potentials in humans--a critical review. Biol Psychol. 1975; 2:237–307.36. Näätänen R, Paavilainen P, Rinne T, Alho K. The mismatch negativity (MMN) in basic research of central auditory processing: a review. Clin Neurophysiol. 2007; 118:2544–2590.
Article37. Näätänen R, Jacobsen T, Winkler I. Memory-based or afferent processes in mismatch negativity (MMN): a review of the evidence. Psychophysiology. 2005; 42:25–32.
Article38. Duncan CC, Barry RJ, Connolly JF, Fischer C, Michie PT, Näätänen R, et al. Event-related potentials in clinical research: guidelines for eliciting, recording, and quantifying mismatch negativity, P300, and N400. Clin Neurophysiol. 2009; 120:1883–1908.
Article39. Winkler I. Interpreting the mismatch negativity. J Psychophysiol. 2007; 21:147–163.
Article40. Ruusuvirta T, Huotilainen M, Fellman V, Näätänen R. Numerical discrimination in newborn infants as revealed by event-related potentials to tone sequences. Eur J Neurosci. 2009; 30:1620–1624.
Article41. Ilvonen T, Kujala T, Kozou H, Kiesiläinen A, Salonen O, Alku P, et al. The processing of speech and non-speech sounds in aphasic patients as reflected by the mismatch negativity (MMN). Neurosci Lett. 2004; 366:235–240.
Article42. Ilvonen TM, Kujala T, Tervaniemi M, Salonen O, Näätänen R, Pekkonen E. The processing of sound duration after left hemisphere stroke: event-related potential and behavioral evidence. Psychophysiology. 2001; 38:622–628.
Article43. Kane NM, Butler SR, Simpson T. Coma outcome prediction using event-related potentials: P(3) and mismatch negativity. Audiol Neurootol. 2000; 5:186–191.
Article44. Kane NM, Curry SH, Butler SR, Cummins BH. Electrophysiological indicator of awakening from coma. Lancet. 1993; 341:688.
Article45. Fischer C, Morlet D, Bouchet P, Luaute J, Jourdan C, Salord F. Mismatch negativity and late auditory evoked potentials in comatose patients. Clin Neurophysiol. 1999; 110:1601–1610.
Article46. Fischer C, Luauté J. Evoked potentials for the prediction of vegetative state in the acute stage of coma. Neuropsychol Rehabil. 2005; 15:372–380.
Article47. Wijnen VJ, Van Boxtel GJ, Eilander HJ, De Gelder B. Mismatch negativity predicts recovery from the vegetative state. Clin Neurophysiol. 2007; 118:597–605.
Article48. Pekkonen E, Huotilainen M, Virtanen J, Sinkkonen J, Rinne T, Ilmoniemi RJ, et al. Age-related functional differences between auditory cortices: a whole-head MEG study. Neuroreport. 1995; 6:1803–1806.
Article49. Pekkonen E, Huotilainen M, Virtanen J, Näätänen R, Ilmoniemi RJ, Erkinjuntti T. Alzheimer's disease affects parallel processing between the auditory cortices. Neuroreport. 1996; 7:1365–1368.
Article50. Escera C, Grau C. Short-term replicability of the mismatch negativity. Electroencephalogr Clin Neurophysiol. 1996; 100:549–554.
Article51. Escera C, Yago E, Polo MD, Grau C. The individual replicability of mismatch negativity at short and long inter-stimulus intervals. Clin Neurophysiol. 2000; 111:546–551.
Article52. Tervaniemi M, Kujala A, Alho K, Virtanen J, Ilmoniemi RJ, Näätänen R. Functional specialization of the human auditory cortex in processing phonetic and musical sounds: a magnetoencephalographic (MEG) study. Neuroimage. 1999; 9:330–336.
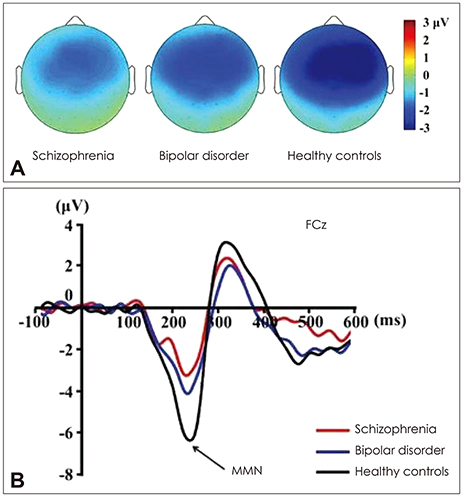
Article53. Kim S, Jeon H, Jang KI, Kim YW, Im CH, Lee SH. Mismatch negativity and cortical thickness in patients with schizophrenia and bipolar disorder. Schizophr Bull. 2019; 45:425–435.
Article54. Kiang M, Braff DL, Sprock J, Light GA. The relationship between preattentive sensory processing deficits and age in schizophrenia patients. Clin Neurophysiol. 2009; 120:1949–1957.
Article55. Light GA, Braff DL. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Arch Gen Psychiatry. 2005; 62:127–136.
Article56. Wynn JK, Sugar C, Horan WP, Kern R, Green MF. Mismatch negativity, social cognition, and functioning in schizophrenia patients. Biol Psychiatry. 2010; 67:940–947.
Article57. Shaikh M, Valmaggia L, Broome MR, Dutt A, Lappin J, Day F, et al. Reduced mismatch negativity predates the onset of psychosis. Schizophr Res. 2012; 134:42–48.
Article58. Higuchi Y, Seo T, Miyanishi T, Kawasaki Y, Suzuki M, Sumiyoshi T. Mismatch negativity and p3a/reorienting complex in subjects with schizophrenia or at-risk mental state. Front Behav Neurosci. 2014; 8:172.
Article59. Bodatsch M, Ruhrmann S, Wagner M, Müller R, Schultze-Lutter F, Frommann I, et al. Prediction of psychosis by mismatch negativity. Biol Psychiatry. 2011; 69:959–966.
Article60. Laton J, Van Schependom J, Gielen J, Decoster J, Moons T, De Keyser J, et al. Single-subject classification of schizophrenia patients based on a combination of oddball and mismatch evoked potential paradigms. J Neurol Sci. 2014; 347:262–267.
Article61. Hegerl U, Juckel G. Intensity dependence of auditory evoked potentials as an indicator of central serotonergic neurotransmission: a new hypothesis. Biol Psychiatry. 1993; 33:173–187.
Article62. Min JA, Lee SH, Lee SY, Chae JH, Lee CU, Park YM, et al. Clinical characteristics associated with different strengths of loudness dependence of auditory evoked potentials (LDAEP) in major depressive disorder. Psychiatry Res. 2012; 200:374–381.
Article63. Hensch T, Wargelius HL, Herold U, Lesch KP, Oreland L, Brocke B. Further evidence for an association of 5-HTTLPR with intensity dependence of auditory-evoked potentials. Neuropsychopharmacology. 2006; 31:2047–2054.
Article64. Linka T, Müller BW, Bender S, Sartory G. The intensity dependence of the auditory evoked N1 component as a predictor of response to Citalopram treatment in patients with major depression. Neurosci Lett. 2004; 367:375–378.
Article65. Linka T, Müller BW, Bender S, Sartory G, Gastpar M. The intensity dependence of auditory evoked ERP components predicts responsiveness to reboxetine treatment in major depression. Pharmacopsychiatry. 2005; 38:139–143.
Article66. Park YM, Lee SH, Kim S, Bae SM. The loudness dependence of the auditory evoked potential (LDAEP) in schizophrenia, bipolar disorder, major depressive disorder, anxiety disorder, and healthy controls. Prog Neuropsychopharmacol Biol Psychiatry. 2010; 34:313–316.
Article67. Yang E, Lee SH, Oh S, Kim S. N100 amplitude slopes in major depressive disorder, bipolar disorder, schizophrenia and normal controls. Korean J Biol Psychiatry. 2009; 16:181–189.68. Gudlowski Y, Ozgürdal S, Witthaus H, Gallinat J, Hauser M, Winter C, et al. Serotonergic dysfunction in the prodromal, first-episode and chronic course of schizophrenia as assessed by the loudness dependence of auditory evoked activity. Schizophr Res. 2009; 109:141–147.
Article69. Park YM, Jung E, Kim HS, Hahn SW, Lee SH. Differences in central serotoninergic transmission among patients with recent onset, sub-chronic, and chronic schizophrenia as assessed by the loudness dependence of auditory evoked potentials. Schizophr Res. 2015; 168:180–184.
Article70. Juckel G. Serotonin: from sensory processing to schizophrenia using an electrophysiological method. Behav Brain Res. 2015; 277:121–124.
Article71. Woolley DW, Shaw E. A biochemical and pharmacological suggestion about certain mental disorders. Proc Natl Acad Sci U S A. 1954; 40:228–231.
Article72. Van Veelen NM, Kahn RS. Dopamine, serotonin, and schizophrenia. Adv Neurol. 1999; 80:425–429.73. Ngan ET, Yatham LN, Ruth TJ, Liddle PF. Decreased serotonin 2A receptor densities in neuroleptic-naive patients with schizophrenia: a PET study using [(18)F]setoperone. Am J Psychiatry. 2000; 157:1016–1018.
Article74. Eastwood SL, Burnet PW, Gittins R, Baker K, Harrison PJ. Expression of serotonin 5-HT(2A) receptors in the human cerebellum and alterations in schizophrenia. Synapse. 2001; 42:104–114.
Article75. Kidmose P, Looney D, Ungstrup M, Rank ML, Mandic DP. A study of evoked potentials from ear-EEG. IEEE Trans Biomed Eng. 2013; 60:2824–2830.
Article76. Rass O, Krishnan G, Brenner CA, Hetrick WP, Merrill CC, Shekhar A, et al. Auditory steady state response in bipolar disorder: relation to clinical state, cognitive performance, medication status, and substance disorders. Bipolar Disord. 2010; 12:793–803.
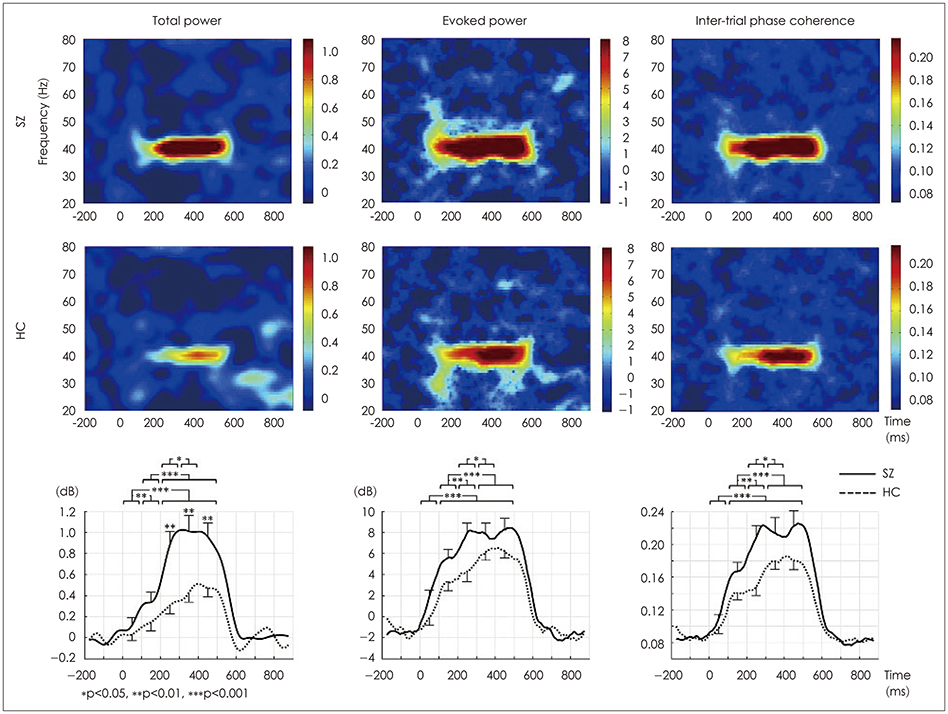
Article77. Thuné H, Recasens M, Uhlhaas PJ. The 40-Hz auditory steady-state response in patients with schizophrenia: a meta-analysis. JAMA Psychiatry. 2016; 73:1145–1153.
Article78. Maran M, Grent T, Uhlhaas PJ. Electrophysiological insights into connectivity anomalies in schizophrenia: a systematic review. Neuropsychiatric Electrophysiology. 2016; 2:6.
Article79. Javitt DC, Sweet RA. Auditory dysfunction in schizophrenia: integrating clinical and basic features. Nat Rev Neurosci. 2015; 16:535–550.
Article80. Leicht G, Andreou C, Polomac N, Lanig C, Schöttle D, Lambert M, et al. Reduced auditory evoked gamma band response and cognitive processing deficits in first episode schizophrenia. World J Biol Psychiatry. 2015; 16:387–397.
Article81. Puvvada KC, Summerfelt A, Du X, Krishna N, Kochunov P, Rowland LM, et al. Delta vs gamma auditory steady state synchrony in schizophrenia. Schizophr Bull. 2018; 44:378–387.
Article82. Kim S, Jang SK, Kim DW, Shim M, Kim YW, Im CH, et al. Cortical volume and 40-Hz auditory-steady-state responses in patients with schizophrenia and healthy controls. Neuroimage Clin. 2019; 22:101732.
Article83. Hong LE, Summerfelt A, McMahon R, Adami H, Francis G, Elliott A, et al. Evoked gamma band synchronization and the liability for schizophrenia. Schizophr Res. 2004; 70:293–302.84. Hall MH, Taylor G, Sham P, Schulze K, Rijsdijk F, Picchioni M, et al. The early auditory gamma-band response is heritable and a putative endophenotype of schizophrenia. Schizophr Bull. 2011; 37:778–787.
Article85. Rass O, Forsyth JK, Krishnan GP, Hetrick WP, Klaunig MJ, Breier A, et al. Auditory steady state response in the schizophrenia, firstdegree relatives, and schizotypal personality disorder. Schizophr Res. 2012; 136:143–149.
Article86. McNally JM, McCarley RW. Gamma band oscillations: a key to understanding schizophrenia symptoms and neural circuit abnormalities. Curr Opin Psychiatry. 2016; 29:202–210.87. Kim T, Thankachan S, McKenna JT, McNally JM, Yang C, Choi JH, et al. Cortically projecting basal forebrain parvalbumin neurons regulate cortical gamma band oscillations. Proc Natl Acad Sci U S A. 2015; 112:3535–3540.
Article88. Steullet P, Cabungcal JH, Monin A, Dwir D, O'Donnell P, Cuenod M, et al. Redox dysregulation, neuroinflammation, and NMDA receptor hypofunction: a “central hub” in schizophrenia pathophysiology? Schizophr Res. 2016; 176:41–51.
Article89. Cohen SM, Tsien RW, Goff DC, Halassa MM. The impact of NMDA receptor hypofunction on GABAergic neurons in the pathophysiology of schizophrenia. Schizophr Res. 2015; 167:98–107.
Article90. Hall MH, Chen CY, Cohen BM, Spencer KM, Levy DL, Öngür D, et al. Genomewide association analyses of electrophysiological endophenotypes for schizophrenia and psychotic bipolar disorders: a preliminary report. Am J Med Genet B Neuropsychiatr Genet. 2015; 168B:151–161.
Article91. Sivarao DV, Chen P, Senapati A, Yang Y, Fernandes A, Benitex Y, et al. 40 Hz auditory steady-state response is a pharmacodynamic biomarker for cortical NMDA receptors. Neuropsychopharmacology. 2016; 41:2232–2240.
Article92. O'Donnell BF, Vohs JL, Krishnan GP, Rass O, Hetrick WP, Morzorati SL. The auditory steady-state response (ASSR): a translational biomarker for schizophrenia. Suppl Clin Neurophysiol. 2013; 62:101–112.93. Bolaños M, Bernat EM, He B, Aviyente S. A weighted small world network measure for assessing functional connectivity. J Neurosci Methods. 2013; 212:133–142.
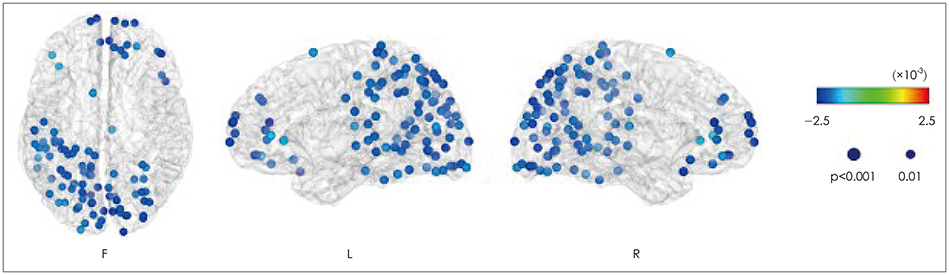
Article94. Fraschini M, Hillebrand A, Demuru M, Didaci L, Marcialis GL. An EEG-based biometric system using eigenvector centrality in resting state brain networks. IEEE Signal Process Lett. 2015; 22:666–670.
Article95. Liu Q, Ganzetti M, Wenderoth N, Mantini D. Detecting large-scale brain networks using EEG: impact of electrode density, head modeling and source localization. Front Neuroinform. 2018; 12:4.
Article96. Micheloyannis S. Graph-based network analysis in schizophrenia. World J Psychiatry. 2012; 2:1–12.
Article97. Yin Z, Li J, Zhang Y, Ren A, Von Meneen KM, Huang L. Functional brain network analysis of schizophrenic patients with positive and negative syndrome based on mutual information of EEG time series. Biomed Signal Process Control. 2017; 31:331–338.
Article98. Shim M, Kim DW, Lee SH, Im CH. Disruptions in small-world cortical functional connectivity network during an auditory oddball paradigm task in patients with schizophrenia. Schizophr Res. 2014; 156:197–203.
Article99. Strelets VB, Novototsky-Vlasov VY, Golikova JV. Cortical connectivity in high frequency beta-rhythm in schizophrenics with positive and negative symptoms. Int J Psychophysiol. 2002; 44:101–115.
Article100. Zhou Y, Liang M, Tian L, Wang K, Hao Y, Liu H, et al. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res. 2007; 97:194–205.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Reduction in Alpha Peak Frequency and Coherence on Quantitative Electroencephalography in Patients with Schizophrenia
- Preliminary Study on Quantitative Sleep EEG Characteristics in Patients with Schizophrenia
- Schizophrenia in Late Life
- The Influence of Negative Emotion to Cortical Activity Induced by Auditory Verbal Imagery in Patients with Schizophrenia
- Treatment-Resistant Schizophrenia : Pathophysiology and Treatment