Korean J Ophthalmol.
2019 Jun;33(3):272-278. 10.3341/kjo.2019.0011.
Refractive Outcomes of 4-Year-old Children after Intravitreal Anti-vascular Endothelial Growth Factor versus Laser Photocoagulation for Retinopathy of Prematurity
- Affiliations
-
- 1Department of Ophthalmology, Institute of Vision Research, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 2Department of Ophthalmology, Institute of Vision Research, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. shhan222@yuhs.ac
- KMID: 2448867
- DOI: http://doi.org/10.3341/kjo.2019.0011
Abstract
- PURPOSE
To compare long-term refractive outcomes associated with intravitreal anti-vascular endothelial growth factor (VEGF) versus laser photocoagulation treatment for retinopathy of prematurity (ROP).
METHODS
A total of 52 eyes from 27 ROP patients treated at two tertiary referral-based hospitals from August 2006 to December 2013 were reviewed. The primary outcome was refractive error measured at the age of 4 years, accounting for within-patient inter-eye correlation. Secondary outcomes included the recurrence rate and treatment complications.
RESULTS
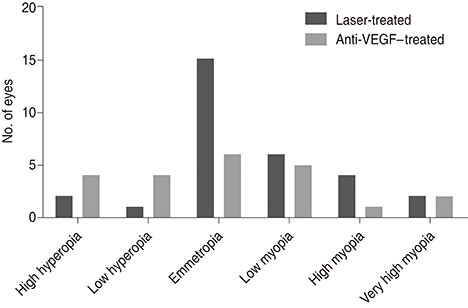
The mean age at refraction was 4.7 ± 0.3 years in the laser group (n = 30) and 4.4 ± 0.3 years in the anti-VEGF group (n = 22). No significant differences were noted in gestational age, birthweight, post-menstrual age at treatment, or ROP stage/zone distribution between groups. Mean spherical equivalent was also not significantly different (−1.0 diopters in the laser group and −0.3 diopters in the injection group, p = 0.603). Clustered regression analysis revealed that only gestational age was significantly correlated with mean spherical equivalent (p < 0.001; 95% confidence interval, −0.007 to −0.002). Recurrence was noted in four eyes (13.3%) in the laser group, but this difference was not significant (p = 0.128). There were no major systemic complications reported in either group.
CONCLUSIONS
Treatment type, whether laser or anti-VEGF injection, does not appear to influence long-term refractive outcomes in ROP. Concern regarding refractive outcomes should not be the most important factor when selecting ROP treatment modality.
Keyword
MeSH Terms
Figure
Reference
-
1. Blencowe H, Lawn JE, Vazquez T, et al. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res. 2013; 74:Suppl 1. 35–49.
Article2. Kushner BJ, Essner D, Cohen IJ, Flynn JT. Retrolental fibroplasia. II. Pathologic correlation. Arch Ophthalmol. 1977; 95:29–38.3. Darlow BA. Retinopathy of prematurity: new developments bring concern and hope. J Paediatr Child Health. 2015; 51:765–770.
Article4. Goggin M, O'Keefe M. Childhood blindness in the Republic of Ireland: a national survey. Br J Ophthalmol. 1991; 75:425–429.
Article5. Hoogerwerf A, Schalij-Delfos NE, van Schooneveld MJ, Termote JU. Incidence of retinopathy of prematurity over the last decade in the Central Netherlands. Neonatology. 2010; 98:137–142.
Article6. Isaza G, Arora S, Bal M, Chaudhary V. Incidence of retinopathy of prematurity and risk factors among premature infants at a neonatal intensive care unit in Canada. J Pediatr Ophthalmol Strabismus. 2013; 50:27–32.
Article7. Tsui I, Chu A. Hot topics in retinopathy of prematurity. Pediatr Ann. 2017; 46:e415–e422.
Article8. Gilbert C, Rahi J, Eckstein M, et al. Retinopathy of prematurity in middle-income countries. Lancet. 1997; 350:12–14.
Article9. Palmer EA, Flynn JT, Hardy RJ, et al. Incidence and early course of retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology. 1991; 98:1628–1640.10. Schaffer DB, Palmer EA, Plotsky DF, et al. Prognostic factors in the natural course of retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology. 1993; 100:230–237.11. Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003; 121:1684–1694.12. Mintz-Hittner HA, Kennedy KA, Chuang AZ. BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011; 364:603–615.
Article13. Repka MX, Tung B, Good WV, et al. Outcome of eyes developing retinal detachment during the Early Treatment for Retinopathy of Prematurity Study (ETROP). Arch Ophthalmol. 2006; 124:24–30.
Article14. Good WV. Early Treatment for Retinopathy of Prematurity Cooperative Group. Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc. 2004; 102:233–248.15. Ahmed AE, Channa R, Durrani J, et al. Early experience with intravitreal bevacizumab combined with laser treatment for retinopathy of prematurity. Middle East Afr J Ophthalmol. 2010; 17:264–267.
Article16. Chung EJ, Kim JH, Ahn HS, Koh HJ. Combination of laser photocoagulation and intravitreal bevacizumab (Avastin) for aggressive zone I retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol. 2007; 245:1727–1730.
Article17. Kong L, Bhatt AR, Demny AB, et al. Pharmacokinetics of bevacizumab and its effects on serum VEGF and IGF-1 in infants with retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2015; 56:956–961.
Article18. Chen SN, Lian I, Hwang YC, et al. Intravitreal anti-vascular endothelial growth factor treatment for retinopathy of prematurity: comparison between ranibizumab and bevacizumab. Retina. 2015; 35:667–674.19. Morin J, Luu TM, Superstein R, et al. Neurodevelopmental outcomes following bevacizumab injections for retinopathy of prematurity. Pediatrics. 2016; 137.
Article20. Abri Aghdam K, Khadamy J, Falavarjani KG, Tsui I. Refractive outcomes following the treatment of retinopathy of prematurity in the anti-VEGF era: a literature review. J AAPOS. 2016; 20:539–540.21. Isaac M, Mireskandari K, Tehrani N. Treatment of type 1 retinopathy of prematurity with bevacizumab versus laser. J AAPOS. 2015; 19:140–144.22. Kang HG, Choi EY, Byeon SH, et al. Anti-vascular endothelial growth factor treatment of retinopathy of prematurity: efficacy, safety, and anatomical outcomes. Korean J Ophthalmol. 2018; 32:451–458.
Article23. International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005; 123:991–999.24. Darlow BA, Ells AL, Gilbert CE, et al. Are we there yet? Bevacizumab therapy for retinopathy of prematurity. Arch Dis Child Fetal Neonatal Ed. 2013; 98:F170–F174.
Article25. Geloneck MM, Chuang AZ, Clark WL, et al. Refractive outcomes following bevacizumab monotherapy compared with conventional laser treatment: a randomized clinical trial. JAMA Ophthalmol. 2014; 132:1327–1333.26. Quinn GE, Dobson V, Kivlin J, et al. Prevalence of myopia between 3 months and 5 1/2 years in preterm infants with and without retinopathy of prematurity. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology. 1998; 105:1292–1300.27. Hwang JH, Lee EH, Kim EA. Retinopathy of prematurity among very-low-birth-weight infants in Korea: incidence, treatment, and risk factors. J Korean Med Sci. 2015; 30:Suppl 1. S88–S94.
Article28. Choi J, Kim JH, Kim SJ, Yu YS. Long-term results of lens-sparing vitrectomy for progressive posterior-type stage 4A retinopathy of prematurity. Korean J Ophthalmol. 2012; 26:277–284.
Article29. Chen YH, Chen SN, Lien RI, et al. Refractive errors after the use of bevacizumab for the treatment of retinopathy of prematurity: 2-year outcomes. Eye (Lond). 2014; 28:1080–1086.
Article30. Kuo HK, Sun IT, Chung MY, Chen YH. Refractive error in patients with retinopathy of prematurity after laser photocoagulation or bevacizumab monotherapy. Ophthalmologica. 2015; 234:211–217.
Article31. Nissenkorn I, Yassur Y, Mashkowski D, et al. Myopia in premature babies with and without retinopathy of prematurity. Br J Ophthalmol. 1983; 67:170–173.
Article32. Chen Y, Fen J, Meng X. Effects of ranibizumab in zone I and zone II retinopathy of prematurity patients. Chin J Ophthalmol. 2015; 31:6–9.33. Huang Q, Zhang Q, Fei P, et al. Ranibizumab injection as primary treatment in patients with retinopathy of prematurity: anatomic outcomes and influencing factors. Ophthalmology. 2017; 124:1156–1164.34. Wu WC, Kuo HK, Yeh PT, et al. An updated study of the use of bevacizumab in the treatment of patients with prethreshold retinopathy of prematurity in Taiwan. Am J Ophthalmol. 2013; 155:150–158.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunohistochemical Study of Transforming Growth Factor-beta2(TGF-beta2) and Vascular Endothelial Growth Factor(VEGF) after Laser Photocoagulation in the Ocular Tissues of White Rats
- A Comparison of Cryotherapy Versus Transscleral Diode Laser Photocoagulation for the Treatment of Retinopathy of Prematurity
- Effect of Laser Photocoagulation and Intravitreal Bevacizumab Injection on Zone I Retinopathy of Prematurity
- Treatment of Acute Retinopathy of Prematurity with Argon Indirect Laser Ophthalmoscope 2nd Report
- Treatment of Exudative Age-Related Macular Degeneration