Korean J Ophthalmol.
2019 Jun;33(3):267-271. 10.3341/kjo.2019.0018.
Treatment of Exposed Hydroxyapatite Orbital Implants Wrapped with a Synthetic Dura Substitute
- Affiliations
-
- 1Department of Ophthalmology, Institute of Vision Research, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. yoonjs@yuhs.ac
- KMID: 2448866
- DOI: http://doi.org/10.3341/kjo.2019.0018
Abstract
- PURPOSE
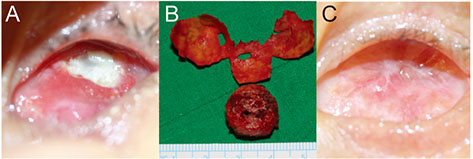
To describe cases of exposed hydroxyapatite (HA) implants wrapped with the synthetic dura substitute Neuro-Patch treated via simple Neuro-Patch removal.
METHODS
The medical records of seven patients who experienced exposure of their HA implant were reviewed. All patients had been enucleated and implanted with HA wrapped with Neuro-Patch. For treatment, Neuro-Patch was removed to the greatest extent possible. After applying local anesthesia with lidocaine, blunt dissection was performed to separate the conjunctiva and Neuro-Patch via the site of exposure. Pressure was applied to the remaining Neuro-Patch with forceps and removed with scissors.
RESULTS
Neuro-Patch was visible at the area of exposure in all patients. No surgery beyond initial Neuro-Patch removal was necessary in six of the seven patients. In five cases, the exposed area began to heal rapidly after Neuro-patch removal without primary closure of the defect. In one case, the Neuro-Patch material and all necrotic tissue was removed aggressively due to inflammation around the orbital implant. Lastly, an infection was noted in one case, prompting complete removal of the Neuro-Patch-wrapped HA implant.
CONCLUSIONS
Wrapping material may hinder implant vascularization. Exposure of HA in wrapped implants can be successfully treated by a simple removal procedure if detected and managed early.
MeSH Terms
Figure
Reference
-
1. Dutton JJ. Coralline hydroxyapatite as an ocular implant. Ophthalmology. 1991; 98:370–377.
Article2. Perry AC. Integrated orbital implants. Adv Ophthalmic Plast Reconstr Surg. 1990; 8:75–81.
Article3. Jordan DR, Klapper SR. Wrapping hydroxyapatite implants. Ophthalmic Surg Lasers. 1999; 30:403–407.4. Lee V, Subak-Sharpe I, Hungerford JL, et al. Exposure of primary orbital implants in postenucleation retinoblastoma patients. Ophthalmology. 2000; 107:940–945.5. Shields CL, Shields JA, De Potter P. Hydroxyapatite orbital implant after enucleation. Experience with initial 100 consecutive cases. Arch Ophthalmol. 1992; 110:333–338.
Article6. Shields JA, Shields CL, De Potter P. Enucleation technique for children with retinoblastoma. J Pediatr Ophthalmol Strabismus. 1992; 29:213–215.
Article7. Yamada S, Aiba T, Endo Y, et al. Creutzfeldt-Jakob disease transmitted by a cadaveric dura mater graft. Neurosurgery. 1994; 34:740–743.
Article8. Mehta JS, Franks WA. The sclera, the prion, and the ophthalmologist. Br J Ophthalmol. 2002; 86:587–592.
Article9. Raul JS, Godard J, Arbez-Gindre F, Czorny A. Use of polyester urethane (Neuro-Patch) as a dural substitute. Prospective study of 70 cases. Neurochirurgie. 2003; 49(2-3 Pt 1):83–89.10. Seiff SR, Chang JS Jr, Hurt MH, Khayam-Bashi H. Polymerase chain reaction identification of human immunodeficiency virus-1 in preserved human sclera. Am J Ophthalmol. 1994; 118:528–530.
Article11. Simonds RJ, Holmberg SD, Hurwitz RL, et al. Transmission of human immunodeficiency virus type 1 from a seronegative organ and tissue donor. N Engl J Med. 1992; 326:726–732.
Article12. Jordan DR, Ells A, Brownstein S, et al. Vicryl-mesh wrap for the implantation of hydroxyapatite orbital implants: an animal model. Can J Ophthalmol. 1995; 30:241–246.13. Jordan DR, Allen LH, Ells A, et al. The use of Vicryl mesh (polyglactin 910) for implantation of hydroxyapatite orbital implants. Ophthalmic Plast Reconstr Surg. 1995; 11:95–99.
Article14. Oestreicher JH, Liu E, Berkowitz M. Complications of hydroxyapatite orbital implants. A review of 100 consecutive cases and a comparison of Dexon mesh (polyglycolic acid) with scleral wrapping. Ophthalmology. 1997; 104:324–329.15. Gudmundsson G, Sogaard I. Complications to the use of vicryl-collagen dural substitute. Acta Neurochir (Wien). 1995; 132:145–147.
Article16. Malliti M, Page P, Gury C, et al. Comparison of deep wound infection rates using a synthetic dural substitute (neuro-patch) or pericranium graft for dural closure: a clinical review of 1 year. Neurosurgery. 2004; 54:599–603.
Article17. El Majdoub F, Lohr M, Maarouf M, et al. Transmigration of fibrino-purulent inflammation and malignant cells into an artificial dura substitute (Neuro-Patch): report of two cases. Acta Neurochir (Wien). 2009; 151:833–835.
Article18. Heimann H, Bechrakis NE, Zepeda LC, et al. Exposure of orbital implants wrapped with polyester-urethane after enucleation for advanced retinoblastoma. Ophthalmic Plast Reconstr Surg. 2005; 21:123–128.
Article19. Kaltreider SA, Newman SA. Prevention and management of complications associated with the hydroxyapatite implant. Ophthalmic Plast Reconstr Surg. 1996; 12:18–31.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Histopathologic Comparison of Vascularization between Dacron and Donor Sclera as Wrapping Material in Hydroxyapatite Implantation
- Management of Exposed Porous Orbital Implant with Autogenous Dermis Graft
- Management of Exposed Hydroxyapatite Implant with Acellular Dermal Allograft
- Tube Erosion with Scleral Melting after Ahmed Valve Implantation Using a Synthetic Dural Substitute
- Hydroxyapatite Implantation using Autogenous Temporalis Muscle Fascia