Ann Hepatobiliary Pancreat Surg.
2019 May;23(2):133-137. 10.14701/ahbps.2019.23.2.133.
Safety and efficacy of peripheral nutrition fluid (MG-TNA®) in patients undergoing surgery for hepatobiliary and pancreatic disease: Results of a phase 4 trial
- Affiliations
-
- 1Division of HBP Surgery & Liver Transplantation, Department of Surgery, Korea University College of Medicine, Seoul, Korea. kimds1@korea.ac.kr
- 2Division of HBP Surgery & Liver Transplantation, Department of Surgery, St. Vincent's Hospital, Suwon, Korea.
- 3Department of Surgery, Inje University Paik Hospital, Ilsan, Korea.
- KMID: 2448766
- DOI: http://doi.org/10.14701/ahbps.2019.23.2.133
Abstract
- BACKGROUNDS/AIMS
Essential nutritional support and nutrition therapy for patients with hepatobiliary and pancreatic diseases undergoing surgery is critical, as it may improve clinical outcome. How to implement rational fluid therapy and nutritional support after surgery and effectively protect organ function is crucial for postoperative recovery. The aim this study was to examine the safety and efficacy of peripheral nutrition fluid (MG-TNA®) in patients undergoing surgery for hepatobiliary and pancreatic disease.
METHODS
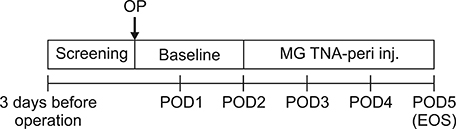
All adult patients undergoing surgery for hepatobiliary and pancreatic disease received peripheral nutrition fluid (MG-TNA®) on the second postoperative day for 3 days. During administration of parenteral nutrition, patients were closely monitored for adverse effects (primary endpoint). Secondary endpoints included nutritional parameters such as serum prealbumin, transferrin, and creatine kinase (CK) levels.
RESULTS
Thirty patients completed the study and were included in the full analysis set. There was no evidence of metabolic complications such as hyperglycemia, azotemia, hypertriglyceridemia, metabolic acidosis and hypokalemia. In addition, there were no adverse effects. There was a significant decrease in serum prealbumin and CK on the third postoperative day (p<0.0001). Although not statistically significant, serum transferrin levels tended to decrease (p=0.0519).
CONCLUSIONS
Administration of peripheral nutrition fluid (MG-TNA®) during postoperative period in patients undergoing surgery for hepatobiliary and pancreatic disease proved to be safe with improvement of the nutritional state of the patient.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Effect of early oral nutrition supplement using Encover in patients undergoing hepato-biliary-pancreatic surgery
Byeong Jun Lee, Joon Seong Park, Hyung Sun Kim, Dong Sup Yoon, Jin Hong Lim
Int J Stem Cells. 2022;26(3):244-250. doi: 10.14701/ahbps.21-152.
Reference
-
1. DuBray BJ Jr, Chapman WC, Anderson CD. Hepatocellular carcinoma: a review of the surgical approaches to management. Mo Med. 2011; 108:195–198.2. Howard L, Ashley C. Nutrition in the perioperative patient. Annu Rev Nutr. 2003; 23:263–282.3. Shiraki M, Nishiguchi S, Saito M, Fukuzawa Y, Mizuta T, Kaibori M, et al. Nutritional status and quality of life in current patients with liver cirrhosis as assessed in 2007–2011. Hepatol Res. 2013; 43:106–112.
Article4. Merli M, Giusto M, Gentili F, Novelli G, Ferretti G, Riggio O, et al. Nutritional status: its influence on the outcome of patients undergoing liver transplantation. Liver Int. 2010; 30:208–214.
Article5. Merli M, Nicolini G, Angeloni S, Riggio O. Malnutrition is a risk factor in cirrhotic patients undergoing surgery. Nutrition. 2002; 18:978–986.
Article6. Fan ST, Lo CM, Lai EC, Chu KM, Liu CL, Wong J. Perioperative nutritional support in patients undergoing hepatectomy for hepatocellular carcinoma. N Engl J Med. 1994; 331:1547–1552.
Article7. Sun Y, Yang Z, Tan H. Perioperative nutritional support and fluid therapy in patients with liver diseases. Hepatobiliary Surg Nutr. 2014; 3:140–148.8. Koretz RL, Avenell A, Lipman TO. Nutritional support for liver disease. Cochrane Database Syst Rev. 2012; (5):CD008344.
Article9. Doig GS, Heighes PT, Simpson F, Sweetman EA. Early enteral nutrition reduces mortality in trauma patients requiring intensive care: a meta-analysis of randomised controlled trials. Injury. 2011; 42:50–56.
Article10. Yi F, Ge L, Zhao J, Lei Y, Zhou F, Chen Z, et al. Meta-analysis: total parenteral nutrition versus total enteral nutrition in predicted severe acute pancreatitis. Intern Med. 2012; 51:523–530.
Article11. Traverso LW, Hashimoto Y. Delayed gastric emptying: the state of the highest level of evidence. J Hepatobiliary Pancreat Surg. 2008; 15:262–269.
Article12. Liu Y, Xue X. Systematic review of peri-operative nutritional support for patients undergoing hepatobiliary surgery. Hepatobiliary Surg Nutr. 2015; 4:304–312.13. Cheung K, Lee SS, Raman M. Prevalence and mechanisms of malnutrition in patients with advanced liver disease, and nutrition management strategies. Clin Gastroenterol Hepatol. 2012; 10:117–125.
Article14. Facciorusso A, Barone M. Glucose intolerance and hepatocellular carcinoma: recent findings for old diseases. Hepatobiliary Surg Nutr. 2014; 3:91–92.15. Martindale RG, McClave SA, Taylor B, Lawson CM. Perioperative nutrition: what is the current landscape? JPEN J Parenter Enteral Nutr. 2013; 37:5 Suppl. 5S–20S.16. Plauth M, Cabré E, Riggio O, Assis-Camilo M, Pirlich M, Kondrup J, et al. ESPEN guidelines on enteral nutrition: liver disease. Clin Nutr. 2006; 25:285–294.
Article17. Mouzaki M, Ng V, Kamath BM, Selzner N, Pencharz P, Ling SC. Enteral energy and macronutrients in end-stage liver disease. JPEN J Parenter Enteral Nutr. 2014; 38:673–681.
Article18. Cahill NE, Murch L, Jeejeebhoy K, McClave SA, Day AG, Wang M, et al. When early enteral feeding is not possible in critically ill patients: results of a multicenter observational study. JPEN J Parenter Enteral Nutr. 2011; 35:160–168.19. Ghabril MS, Aranda-Michel J, Scolapio JS. Metabolic and catheter complications of parenteral nutrition. Curr Gastroenterol Rep. 2004; 6:327–334.
Article20. Demirer S, Sapmaz A, Karaca AS, Kepenekci I, Aydintug S, Balci D, et al. Effects of postoperative parenteral nutrition with different lipid emulsions in patients undergoing major abdominal surgery. Ann Surg Treat Res. 2016; 91:309–315.
Article21. Bozzetti F, Arends J, Lundholm K, Micklewright A, Zurcher G, Muscaritoli M. ESPEN. ESPEN guidelines on parenteral nutrition: non-surgical oncology. Clin Nutr. 2009; 28:445–454.
Article22. Zhu X, Wu Y, Qiu Y, Jiang C, Ding Y. Effect of parenteral fish oil lipid emulsion in parenteral nutrition supplementation combined with enteral nutrition support in patients undergoing pancreaticoduodenectomy. JPEN J Parenter Enteral Nutr. 2013; 37:236–242.
Article23. Jiang JW, Ren ZG, Chen LY, Jiang L, Xie HY, Zhou L, et al. Enteral supplementation with glycyl-glutamine improves intestinal barrier function after liver transplantation in rats. Hepatobiliary Pancreat Dis Int. 2011; 10:380–385.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy of Topiramate Using Lower Dose and Slower Dose-Titration in Refractory Partial Epilepsies: A Multicenter Open Clinical Trial
- Systematic Review on the Efficacy and Safety of Erenumab for the Prevention of Migraine
- An Open, Randomized, Multicenter Comparative Clinical Trial of Lamotrigine and Carbamazepine as Initial Monotherapy in Previously Untreated Epilepsies
- Clinical Efficacy and Safety of Intravenous levofloxacin in Patients of Abdominal Operati
- Endoscopic Management of Pancreatic Fluid Collections in Children