Ann Hepatobiliary Pancreat Surg.
2019 May;23(2):109-114. 10.14701/ahbps.2019.23.2.109.
Molecular classification of hepatocellular adenoma: A single-center experience
- Affiliations
-
- 1Division of Hepatobiliary Surgery and Liver Transplantation, Department of Surgery, Ajou University School of Medicine, Suwon, Korea. wanghj@ajou.ac.kr
- 2Department of Hepatobiliary Surgery, Jiangxi Cancer Center, Nanchang, China.
- 3Department of Pathology, Ajou University School of Medicine, Suwon, Korea.
- KMID: 2448762
- DOI: http://doi.org/10.14701/ahbps.2019.23.2.109
Abstract
- BACKGROUNDS/AIMS
Hepatocellular adenoma (HCA) is a rare benign tumor that has a risk of malignant transformation into hepatocellular carcinoma (HCC) and bleeding. The aim of this study was to analyze the characteristics of HCA by performing molecular classification.
METHODS
We retrospectively collected data from nine patients who were diagnosed with HCA from 1995 to 2016. The patients underwent liver surgery due to the existence of clinical symptoms. Immunohistochemical (IHC) staining was performed to classify the subgroups of HCA.
RESULTS
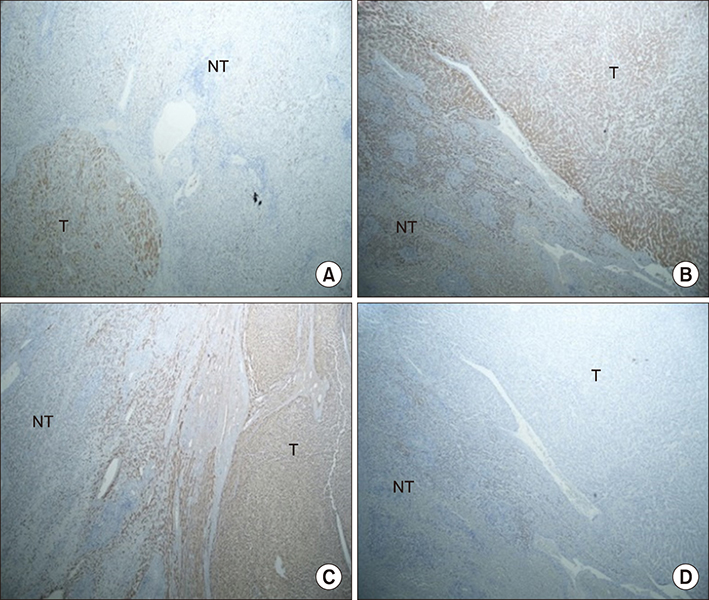
Four patients with both β-catenin and inflammation were classified as β-IHCA. Two patients were defined as β-HCA. Two patients were classified as HHCA. Only one patient was defined as IHCA. None of the patients had unclassified HCA. Seven of nine patients had a malignant transformation. By comparing the characteristics of HCA between two groups, we found the mean tumor size in the malignant transformation group was greater than the non-malignant transformation group.
CONCLUSIONS
Taken together, the mean tumor size and activation of catenin β1 mutation status might be the risk factors for the malignant transformation of HCA into HCC. Moreover, IHCA without the catenin β1 mutation could also have a possibility of malignant transformation into HCC.
Keyword
MeSH Terms
Figure
Reference
-
1. Rooks JB, Ory HW, Ishak KG, Strauss LT, Greenspan JR, Hill AP, et al. Epidemiology of hepatocellular adenoma. The role of oral contraceptive use. JAMA. 1979; 242:644–648.
Article2. Heinemann LA, Weimann A, Gerken G, Thiel C, Schlaud M, DoMinh T. Modern oral contraceptive use and benign liver tumors: the German Benign Liver Tumor Case-Control Study. Eur J Contracept Reprod Health Care. 1998; 3:194–200.
Article3. Sasaki M, Nakanuma Y. Overview of hepatocellular adenoma in Japan. Int J Hepatol. 2012; 2012:648131.
Article4. Matsumoto Y, Yamabe S, Sugishima T, Geronazzo D. Perception of oral contraceptives among women of reproductive age in Japan: a comparison with the USA and France. J Obstet Gynaecol Res. 2011; 37:887–892.
Article5. Svrcek M, Jeannot E, Arrivé L, Poupon R, Fromont G, Fléjou JF, et al. Regressive liver adenomatosis following androgenic progestin therapy withdrawal: a case report with a 10-year follow-up and a molecular analysis. Eur J Endocrinol. 2007; 156:617–621.
Article6. Reddy SK, Kishnani PS, Sullivan JA, Koeberl DD, Desai DM, Skinner MA, et al. Resection of hepatocellular adenoma in patients with glycogen storage disease type Ia. J Hepatol. 2007; 47:658–663.
Article7. Ozenne V, Paradis V, Vullierme MP, Vilgrain V, Leblanc T, Belghiti J, et al. Liver tumours in patients with Fanconi anaemia: a report of three cases. Eur J Gastroenterol Hepatol. 2008; 20:1036–1039.
Article8. Schmidt J. Systematic review of haemorrhage and rupture of hepatocellular adenomas. Br J Surg. 2012; 99:911–916.9. Bioulac-Sage P, Laumonier H, Couchy G, Le Bail B, Sa Cunha A, Rullier A, et al. Hepatocellular adenoma management and phenotypic classification: the Bordeaux experience. Hepatology. 2009; 50:481–489.
Article10. Dokmak S, Paradis V, Vilgrain V, Sauvanet A, Farges O, Valla D, et al. A single-center surgical experience of 122 patients with single and multiple hepatocellular adenomas. Gastroenterology. 2009; 137:1698–1705.
Article11. Deneve JL, Pawlik TM, Cunningham S, Clary B, Reddy S, Scoggins CR, et al. Liver cell adenoma: a multicenter analysis of risk factors for rupture and malignancy. Ann Surg Oncol. 2009; 16:640–648.
Article12. Farges O, Ferreira N, Dokmak S, Belghiti J, Bedossa P, Paradis V. Changing trends in malignant transformation of hepatocellular adenoma. Gut. 2011; 60:85–89.
Article13. Bacq Y, Jacquemin E, Balabaud C, Jeannot E, Scotto B, Branchereau S, et al. Familial liver adenomatosis associated with hepatocyte nuclear factor 1alpha inactivation. Gastroenterology. 2003; 125:1470–1475.14. Bluteau O, Jeannot E, Bioulac-Sage P, Marqués JM, Blanc JF, Bui H, et al. Bi-allelic inactivation of TCF1 in hepatic adenomas. Nat Genet. 2002; 32:312–315.
Article15. Jeannot E, Mellottee L, Bioulac-Sage P, Balabaud C, Scoazec JY, Tran Van Nhieu J, et al. Spectrum of HNF1A somatic mutations in hepatocellular adenoma differs from that in patients with MODY3 and suggests genotoxic damage. Diabetes. 2010; 59:1836–1844.
Article16. Zucman-Rossi J, Jeannot E, Nhieu JT, Scoazec JY, Guettier C, Rebouissou S, et al. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology. 2006; 43:515–524.
Article17. Nault JC, Couchy G, Balabaud C, Morcrette G, Caruso S, Blanc JF, et al. Molecular classification of hepatocellular adenoma associates with risk factors, bleeding, and malignant transformation. Gastroenterology. 2017; 152:880–894.18. Pilati C, Letouzé E, Nault JC, Imbeaud S, Boulai A, Calderaro J, et al. Genomic profiling of hepatocellular adenomas reveals recurrent FRK-activating mutations and the mechanisms of malignant transformation. Cancer Cell. 2014; 25:428–441.
Article19. Shafizadeh N, Genrich G, Ferrell L, Kakar S. Hepatocellular adenomas in a large community population, 2000 to 2010: reclassification per current World Health Organization classification and results of long-term follow-up. Hum Pathol. 2014; 45:976–983.
Article20. Davis M, Portmann B, Searle M, Wright R, Williams R. Histological evidence of carcinoma in a hepatic tumour associated with oral contraceptives. Br Med J. 1975; 4:496–498.
Article21. Stoot JH, Coelen RJ, De Jong MC, Dejong CH. Malignant transformation of hepatocellular adenomas into hepatocellular carcinomas: a systematic review including more than 1600 adenoma cases. HPB (Oxford). 2010; 12:509–522.
Article22. Gorayski P, Thompson CH, Subhash HS, Thomas AC. Hepatocellular carcinoma associated with recreational anabolic steroid use. Br J Sports Med. 2008; 42:74–75. discussion 75.
Article23. Labrune P, Trioche P, Duvaltier I, Chevalier P, Odièvre M. Hepatocellular adenomas in glycogen storage disease type I and III: a series of 43 patients and review of the literature. J Pediatr Gastroenterol Nutr. 1997; 24:276–279.
Article24. Franco LM, Krishnamurthy V, Bali D, Weinstein DA, Arn P, Clary B, et al. Hepatocellular carcinoma in glycogen storage disease type Ia: a case series. J Inherit Metab Dis. 2005; 28:153–162.
Article25. Baheti AD, Yeh MM, O'Malley R, Lalwani N. Malignant transformation of hepatic adenoma in glycogen storage disease type-1a: report of an exceptional case diagnosed on surveillance imaging. J Clin Imaging Sci. 2015; 5:47.
Article26. Cassiman D, Libbrecht L, Verslype C, Meersseman W, Troisi R, Zucman-Rossi J, et al. An adult male patient with multiple adenomas and a hepatocellular carcinoma: mild glycogen storage disease type Ia. J Hepatol. 2010; 53:213–217.
Article27. Calderaro J, Labrune P, Morcrette G, Rebouissou S, Franco D, Prévot S, et al. Molecular characterization of hepatocellular adenomas developed in patients with glycogen storage disease type I. J Hepatol. 2013; 58:350–357.
Article28. Bioulac-Sage P, Rebouissou S, Thomas C, Blanc JF, Saric J, Sa Cunha A, et al. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology. 2007; 46:740–748.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hepatocellular Carcinoma Arising in Hepatocellular Adenoma
- Hepatocellular adenomas: recent updates
- Hepatocellular Carcinoma Arising from Hepatocellular Adenoma in an Elderly Male Patient
- Benign hepatocellular nodules of healthy liver: focal nodular hyperplasia and hepatocellular adenoma
- Hepatocellular Adenoma and Focal Nodular Hyperplasia