Allergy Asthma Immunol Res.
2019 Jul;11(4):519-528. 10.4168/aair.2019.11.4.519.
Add-on Tiotropium in Chinese Patients With Moderate Asthma: A Pooled Subgroup Analysis of MezzoTinA-Asthma 1 and 2
- Affiliations
-
- 1Department of Pulmonary and Critical Care Medicine, China-Japan Friendship Hospital, Beijing, China. jiangtao_l@263.net
- 2Department of Pulmonary and Critical Care Medicine, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China.
- 3Department of Pulmonary Diseases, the First Hospital of China Medical University, Shenyang, China.
- 4Department of Pulmonary Diseases, Xinqiao Hospital, Chongqing, China.
- 5Department of Pulmonary Diseases, the Second Xiangya Hospital of Central South University, Changsha, China.
- 6Department of Pulmonary Diseases, Zhongshan Hospital, Fudan University, Shanghai, China.
- 7Department of Pulmonary Diseases, Capital Medical University Affiliated Beijing Friendship Hospital, Beijing, China.
- 8Department of Pulmonary and Critical Care Medicine, Capital Medical University Affiliated Anzhen Hospital, Capital Medical University, Beijing, China.
- 9Department of Pulmonary Diseases, First Affiliated Hospital of Kunming Medical University, Kunming, China.
- 10Department of Pulmonary Diseases, Nanjing Chest Hospital, Nanjing, China.
- 11Boehringer Ingelheim, Shanghai, China.
- KMID: 2448754
- DOI: http://doi.org/10.4168/aair.2019.11.4.519
Abstract
- PURPOSE
Asthma affects approximately 30 million patients in China; however, tiotropium data for Chinese patients is limited. This study aimed to assess the efficacy and safety of tiotropium in Chinese patients with moderate symptomatic asthma.
METHODS
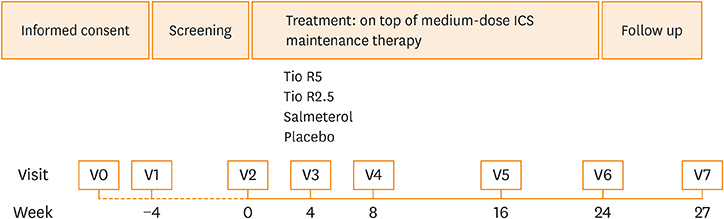
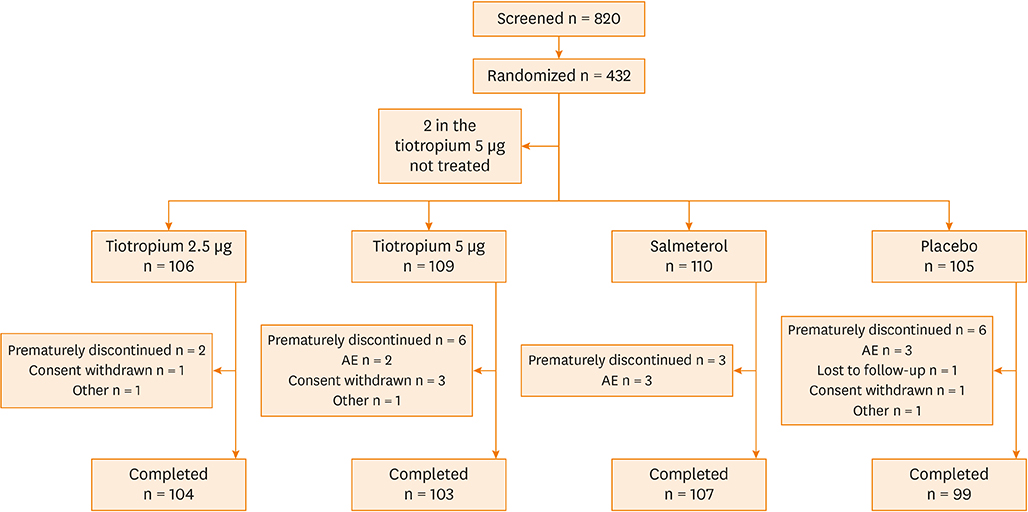
A post hoc subgroup analysis was conducted on 430 Chinese patients pooled from two 24-week, replicate phase 3 trials (NCT01172808 and NCT01172821), in which they received once-daily tiotropium 2.5 µg (Tio R2.5) or 5 µg (Tio R5) (n = 106 or 109, respectively), twice-daily salmeterol 50 µg (Sal 50) (n = 110), or placebo (n = 105), while maintaining inhaled corticosteroids (ICS). The co-primary endpoints assessed in week 24 were forced expiratory volume in 1 second (FEV1) peak0-3h response, trough FEV1 response, and responder rate as assessed using the Asthma Control Questionnaire (ACQ).
RESULTS
For both FEV1 peak0-3h responses and trough FEV1 responses, the mean treatment differences were greater for Tio R2.5, Tio R5, and Sal 50 compared with placebo at 0.249 L, 0.234 L, and 0.284 L, and 0.172 L, 0.180 L, and 0.164 L, respectively (P< 0.001). The ACQ responder rate in placebo, Tio R2.5, Tio R5, and Sal 50 was 58.7%, 62.3%, 59.3%, and 69.1%, respectively. Furthermore, 11 (2.6%) of 430 patients had serious adverse events (Tio R5, n = 4; Tio R2.5, n = 1; Sal 50, n = 1; and placebo, n = 5).
CONCLUSIONS
Once-daily tiotropium, as add-on to medium-dose ICS, was effective and well tolerated for Chinese patients with moderate symptomatic asthma, consistent with the main analysis.
Keyword
MeSH Terms
Figure
Reference
-
1. To T, Stanojevic S, Moores G, Gershon AS, Bateman ED, Cruz AA, et al. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health. 2012; 12:204.
Article2. Su N, Lin J, Liu G, Chen P, Zhou X, Wan H, et al. An epidemiological survey of current asthma control status in China. Zhonghua Nei Ke Za Zhi. 2014; 53:601–606.3. Global Strategy for Asthma Management and Prevention. 2018 Global initiative for asthma [Internet]. Fontana (WI): Global Initiative for Asthma;2018. cited 2019 Jan 30. Available from: http://ginasthma.org/.4. Dahl R. Systemic side effects of inhaled corticosteroids in patients with asthma. Respir Med. 2006; 100:1307–1317.
Article5. Peters SP, Kunselman SJ, Icitovic N, Moore WC, Pascual R, Ameredes BT, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010; 363:1715–1726.6. Bateman ED, Kornmann O, Schmidt P, Pivovarova A, Engel M, Fabbri LM. Tiotropium is noninferior to salmeterol in maintaining improved lung function in B16-Arg/Arg patients with asthma. J Allergy Clin Immunol. 2011; 128:315–322.
Article7. Kerstjens HA, Disse B, Schröder-Babo W, Bantje TA, Gahlemann M, Sigmund R, et al. Tiotropium improves lung function in patients with severe uncontrolled asthma: a randomized controlled trial. J Allergy Clin Immunol. 2011; 128:308–314.8. Kerstjens HA, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, et al. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med. 2012; 367:1198–1207.
Article9. Paggiaro P, Halpin DM, Buhl R, Engel M, Zubek VB, Blahova Z, et al. The effect of tiotropium in symptomatic asthma despite low- to medium-dose inhaled corticosteroids: a randomized controlled trial. J Allergy Clin Immunol Pract. 2016; 4:104–113.e2.
Article10. Kew KM, Dahri K. Long-acting muscarinic antagonists (LAMA) added to combination long-acting beta2-agonists and inhaled corticosteroids (LABA/ICS) versus LABA/ICS for adults with asthma. Cochrane Database Syst Rev. 2016; CD011721.
Article11. Kerstjens HA, Casale TB, Bleecker ER, Meltzer EO, Pizzichini E, Schmidt O, et al. Tiotropium or salmeterol as add-on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double-blind, placebo-controlled, parallel-group, active-comparator, randomised trials. Lancet Respir Med. 2015; 3:367–376.12. Rhee H, Love T, Mammen J. Comparing Asthma Control Questionnaire (ACQ) and National Asthma Education and Prevention Program (NAEPP) asthma control criteria. Ann Allergy Asthma Immunol. 2019; 122:58–64.
Article13. Bateman ED, Esser D, Chirila C, Fernandez M, Fowler A, Moroni-Zentgraf P, et al. Magnitude of effect of asthma treatments on Asthma Quality of Life Questionnaire and Asthma Control Questionnaire scores: Systematic review and network meta-analysis. J Allergy Clin Immunol. 2015; 136:914–922.
Article14. Aalbers R, Park HS. Positioning of long-acting muscarinic antagonists in the management of asthma. Allergy Asthma Immunol Res. 2017; 9:386–393.
Article15. Halpin DM, Kaplan AG, Russell RK. Why choose tiotropium for my patient? A comprehensive review of actions and outcomes versus other bronchodilators. Respir Med. 2017; 128:28–41.
Article16. Buhl R, FitzGerald JM, Busse WW. Tiotropium add-on to inhaled corticosteroids versus addition of long-acting β2-agonists for adults with asthma. Respir Med. 2018; 143:82–90.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Positioning of Long-Acting Muscarinic Antagonists in the Management of Asthma
- The role of tiotropium in the management of asthma
- A Comparison of International Guidelines for Pediatric Asthma Pharmacotherapy
- Add-on Therapy for Symptomatic Asthma despite Long-Acting Beta-Agonists/Inhaled Corticosteroid
- Expression of Muscarinic Receptors and the Effect of Tiotropium Bromide in Aged Mouse Model of Chronic Asthma