Nutr Res Pract.
2018 Dec;12(6):479-485. 10.4162/nrp.2018.12.6.479.
Anti-inflammatory effects of Agar free-Gelidium amansii (GA) extracts in high-fat diet-induced obese mice
- Affiliations
-
- 1Department of Food Science and Nutrition, Jeju National University, Jeju 63243, Korea.
- 2Cancer and Diabetes institute, Gachon University, Seongnam, Gyeonggi 13120, Korea.
- 3Department of Food and Nutrition & Research Institute of Obesity Sciences, Sungshin Women's University, 76ga-55, Dobong-ro, Gangbuk-gu, Seoul 01133, Korea. mlee@sungshin.ac.kr
- KMID: 2448692
- DOI: http://doi.org/10.4162/nrp.2018.12.6.479
Abstract
- BACKGROUND/OBJECTIVES
Gelidium amansii (GA) contains plenty of agars and various biological substances, which make them a popular functional food to control body weight in previous studies. Unlike previous studies focused on agar in GA, objectives of this study were to investigate the effects of agar-free GA extract (AfGAE) on preventive and treatment models by using diets-induced obese (DIO) C57BL/6J mice.
MATERIALS/METHODS
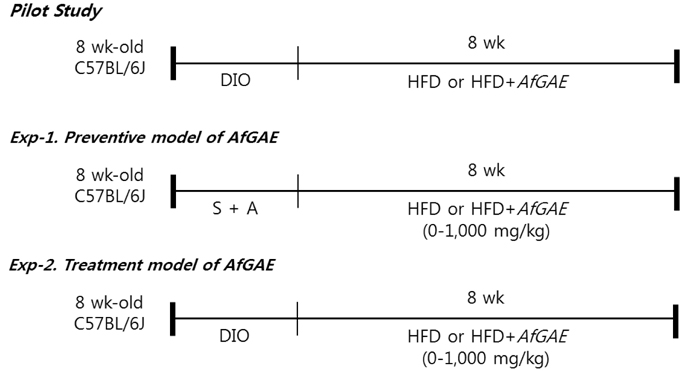
AfGAE were used to test their effects on the prevention (Exp-1) and treatment (Exp-2) against obesity after pilot study in DIO mice. The weight changes of the body and fat tissues and protein expression related to lipid metabolism and inflammation as well as plasma lipid profile and insulin were detected.
RESULTS
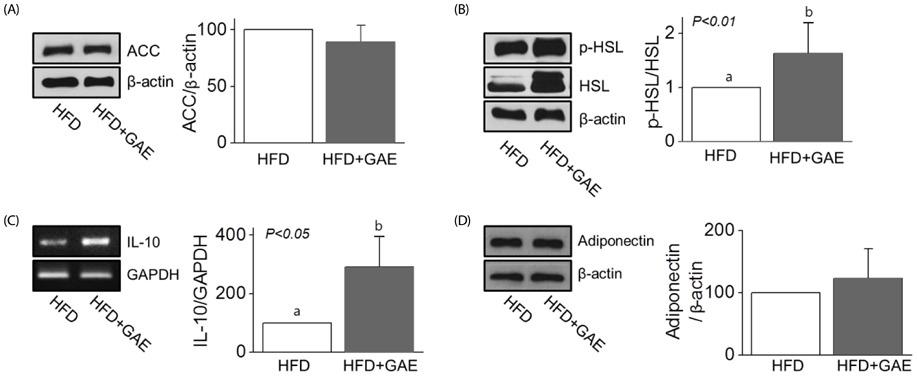
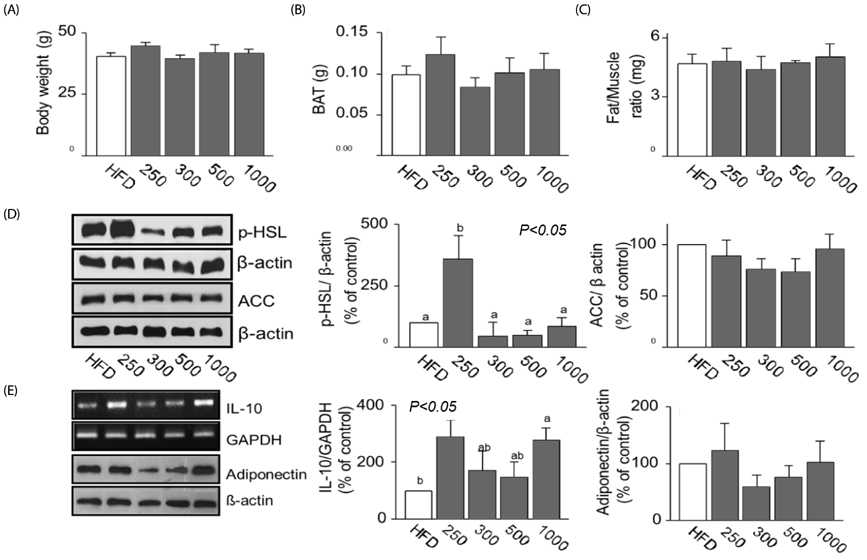
Although AfGAE did not prevent long-term DIO, it did increase the levels of anti-inflammatory cytokine production and lipolysis protein. We further evaluated various doses of AfGAE in preventive and treatment models. As a result, our findings suggested that an AfGAE administration as a preventive model might be a better approach to achieve its anti-inflammatory and lipolysis-promoting effects in DIO mice.
CONCLUSION
Although future studies to investigate the target materials such as polyphenols in AfGAE are required, the result suggests that GA without agar might be a therapeutic tool to improve health conditions related to inflammation.
Keyword
MeSH Terms
Figure
Reference
-
1. Park EY, Jeong SM, Kim YJ, Lee DH. Review on hydrolysis methods of the macroalgae for production of bioethanol. J Korea Soc Waste Manage. 2012; 29:323–333.2. Mohamed S, Hashim SN, Rahman HA. Seaweeds: a sustainable functional food for complementary and alternative therapy. Trends Food Sci Technol. 2012; 23:83–96.
Article3. Xu N, Fan X, Yan X, Li X, Niu R, Tseng CK. Antibacterial bromophenols from the marine red alga Rhodomela confervoides. Phytochemistry. 2003; 62:1221–1224.
Article4. Jung WK, Choi I, Oh S, Park SG, Seo SK, Lee SW, Lee DS, Heo SJ, Jeon YJ, Je JY, Ahn CB, Kim JS, Oh KS, Kim YM, Moon C, Choi IW. Anti-asthmatic effect of marine red alga (Laurencia undulata) polyphenolic extracts in a murine model of asthma. Food Chem Toxicol. 2009; 47:293–297.
Article5. Duan XJ, Zhang WW, Li XM, Wang BG. Evaluation of antioxidant property of extract and fractions obtained from a red alga, Polysiphonia urceolata. Food Chem. 2006; 95:37–43.
Article6. Wang BG, Zhang WW, Duan XJ, Li XM. In vitro antioxidative activities of extract and semi-purified fractions of the marine red alga, Rhodomela confervoides (Rhodomelaceae). Food Chem. 2009; 113:1101–1105.
Article7. Park JI, Woo HC, Lee JH. Production of bio-energy from marine algae: status and perspectives. Korean Chem Eng Res. 2008; 46:833–844.8. Kang MC, Kang N, Kim SY, Lima IS, Ko SC, Kim YT, Kim YB, Jeung HD, Choi KS, Jeon YJ. Popular edible seaweed, Gelidium amansii prevents against diet-induced obesity. Food Chem Toxicol. 2016; 90:181–187.
Article9. Kim MS, Kim JY, Choi WH, Lee SS. Effects of seaweed supplementation on blood glucose concentration, lipid profile, and antioxidant enzyme activities in patients with type 2 diabetes mellitus. Nutr Res Pract. 2008; 2:62–67.
Article10. Seo MJ, Lee OH, Choi HS, Lee BY. Extract from edible red seaweed (Gelidium amansii) inhibits lipid accumulation and ROS production during differentiation in 3T3-L1 cells. Prev Nutr Food Sci. 2012; 17:129–135.
Article11. Yang TH, Yao HT, Chiang MT. Red algae (Gelidium amansii) reduces adiposity via activation of lipolysis in rats with diabetes induced by streptozotocin-nicotinamide. J Food Drug Anal. 2015; 23:758–765.
Article12. Maeda H, Yamamoto R, Hirao K, Tochikubo O. Effects of agar (kanten) diet on obese patients with impaired glucose tolerance and type 2 diabetes. Diabetes Obes Metab. 2005; 7:40–46.
Article13. Kang MC, Kang N, Ko SC, Kim YB, Jeon YJ. Anti-obesity effects of seaweeds of Jeju Island on the differentiation of 3T3-L1 preadipocytes and obese mice fed a high-fat diet. Food Chem Toxicol. 2016; 90:36–44.
Article14. Samarakoon KW, Elvitigala DAS, Lakmal HHC, Kim YM, Jeon YJ. Future prospects and health benefits of functional ingredients from marine bio-resources: a review. Fish Aquat Sci. 2014; 17:275–290.
Article15. Kim J, Kim HJ, Lee M. The suppressive effect of Gelidium amansi-EtOH extracts on the adipogenesis with MAPK signals in adipocytes with or without macrophages. Food Sci Biotechnol. 2017; 26:1715–1723.
Article16. Lee SM, Yu BJ, Kim YM, Choi SJ, Ha JM, Lee JH. Production of bio-ethanol from agar using Saccharomyces cerevisiae. J Korean Ind Eng Chem. 2009; 20:290–295.17. Kim SY, Wi HR, Choi S, Ha TJ, Lee BW, Lee M. Inhibitory effect of anthocyanin-rich black soybean testa (Glycine max (L.) Merr.) on the inflammation-induced adipogenesis in a DIO mouse model. J Funct Foods. 2015; 14:623–633.
Article18. Na SY, Myung SJ. Obesity and colorectal cancer. Korean J Gastroenterol. 2012; 59:16–26.
Article19. Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005; 46:2347–2355.
Article20. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003; 112:1796–1808.
Article21. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003; 112:1821–1830.
Article22. Moro CO, Basile G. Obesity and medicinal plants. Fitoterapia. 2000; 71:Suppl 1. S73–S82.
Article23. Kang JH, Lee HA, Kim HJ, Han JS. Gelidium amansii extract ameliorates obesity by down-regulating adipogenic transcription factors in diet-induced obese mice. Nutr Res Pract. 2017; 11:17–24.
Article24. Shoelson SE, Lee J, Yuan M. Inflammation and the IKK β/I κ B/NF-κ B axis in obesity- and diet-induced insulin resistance. Int J Obes Relat Metab Disord. 2003; 27:Suppl 3. S49–S52.25. Okuda H, Morimoto C, Tsujita T. Effect of substrates on the cyclic AMP-dependent lipolytic reaction of hormone-sensitive lipase. J Lipid Res. 1994; 35:1267–1273.
Article26. Park MK, Jung U, Roh C. Fucoidan from marine brown algae inhibits lipid accumulation. Mar Drugs. 2011; 9:1359–1367.
Article27. Heo SJ, Cha SH, Lee KW, Jeon YJ. Antioxidant activities of red algae from Jeju Island. Algae. 2006; 21:149–156.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gelidium amansii extract ameliorates obesity by down-regulating adipogenic transcription factors in diet-induced obese mice
- Anti-inflammatory and anti-diabetic effects of brown seaweeds in high-fat diet-induced obese mice
- Effects of resveratrol on the insulin signaling pathway of obese mice
- Expression of eotaxin in 3T3-L1 adipocytes and the effects of weight loss in high-fat diet induced obese mice
- The Effects of the Sasa Borealis Leaves Extract on Plasma Adiponectin, Resistin, C-Reactive Protein and Homocysteine Levels in High Fat Diet-Induced Obese C57/BL6J Mice