Clin Exp Otorhinolaryngol.
2019 May;12(2):169-175. 10.21053/ceo.2018.00983.
Effect of Ginkgo Biloba Extract on N-Methyl-D-Aspartic Acid Receptor Subunit 2B Expression in a Salicylate-Induced Ototoxicity Model
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, SMG-SNU Boramae Medical Center, Seoul National University College of Medicine, Seoul, Korea. yhkiment@gmail.com
- 2Department of Pathology, SMG-SNU Boramae Medical Center, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2447425
- DOI: http://doi.org/10.21053/ceo.2018.00983
Abstract
OBJECTIVES
.: Sodium salicylate (SS) is well known for its ototoxic properties that induce functional and morphological changes in the cochlea and brain. Ginkgo biloba extract (GBE) has been widely used for treatment of various neurodegenerative diseases; however, its effects on salicylate-induced ototoxicity remain unclear. Herein, we examined the effects of EGb 761 (EGb), a standard form of GBE, on the plasticity of the N-methyl-D-aspartate receptor subunit 2B (GluN2B) in the inferior colliculus (IC) following SS administration.
METHODS
.: Seven-week-old Sprague Dawley rats (n=24) were randomly allocated to control, SS, EGb, and EGb+SS groups. The SS group received a single intraperitoneal SS injection (350 mg/kg), the EGb group received EGb orally for 5 consecutive days (40 mg/kg), and the EGb+SS group received EGb for 5 consecutive days, followed by an SS injection. The auditory brainstem responses (ABRs) were assessed at baseline and 2 hours after SS administration. GluN2B expression was examined by Western blot and immunohistochemistry.
RESULTS
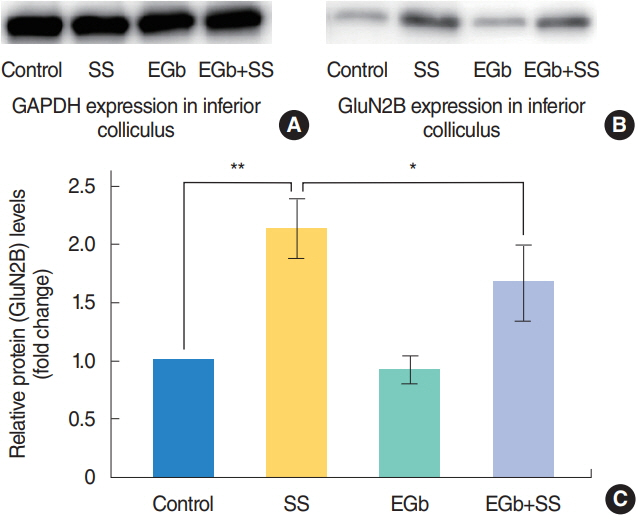
.: There were no significant differences in ABR threshold shifts among the groups. The expression of the GluN2B protein normalized by which of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was significantly lower in the EGb+SS group, as compared to the SS group (P=0.012). Weak and diffused GluN2B immunoreactivity was detected in the IC neural cells of the EGb+SS group, while those of the SS group exhibited strong and diffused GluN2B positivity.
CONCLUSION
.: EGb may play a role in regulating the GluN2B expression in the IC of salicylate-induced ototoxicity model.
MeSH Terms
-
Blotting, Western
Brain
Cochlea
Evoked Potentials, Auditory, Brain Stem
Ginkgo biloba*
Glyceraldehyde 3-Phosphate
Immunohistochemistry
Inferior Colliculi
N-Methylaspartate*
Neurodegenerative Diseases
Oxidoreductases
Plastics
Rats, Sprague-Dawley
Sodium Salicylate
Glyceraldehyde 3-Phosphate
N-Methylaspartate
Oxidoreductases
Plastics
Sodium Salicylate
Figure
Reference
-
1. Sinha GP, Sabri F, Dimitriadis EK, Iwasa KH. Organization of membrane motor in outer hair cells: an atomic force microscopic study. Pflugers Arch. 2010; Feb. 459(3):427–39.
Article2. Guitton MJ, Caston J, Ruel J, Johnson RM, Pujol R, Puel JL. Salicylate induces tinnitus through activation of cochlear NMDA receptors. J Neurosci. 2003; May. 23(9):3944–52.
Article3. Parsons MP, Raymond LA. Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron. 2014; Apr. 82(2):279–93.
Article4. Oestreicher E, Arnold W, Felix D. Neurotransmission of the cochlear inner hair cell synapse-implications for inner ear therapy. In : Felix D, Oestreicher E, editors. Rational pharmacotherapy of the inner ear. Basel: Karger Publishers;2002. p. 131–9.5. Imsuwansri T, Hoare DJ, Phaisaltuntiwongs W, Srisubat A, Snidvongs K. Glutamate receptor antagonists for tinnitus. Cochrane Database Syst Rev. 2016; Oct. 10(CD012391):
Article6. Mitchell JA, Akarasereenont P, Thiemermann C, Flower RJ, Vane JR. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci U S A. 1993; Dec. 90(24):11693–7.
Article7. Hu SS, Mei L, Chen JY, Huang ZW, Wu H. Expression of immediateearly genes in the inferior colliculus and auditory cortex in salicylateinduced tinnitus in rat. Eur J Histochem. 2014; Mar. 58(1):2294.
Article8. Szasz BK, Lenkey N, Barth AM, Mike A, Somogyvari Z, Farkas O, et al. Converging effects of Ginkgo biloba extract at the level of transmitter release, NMDA and sodium currents and dendritic spikes. Planta Med. 2008; Aug. 74(10):1235–9.9. Dave JR, Williams AJ, Moffett JR, Koenig ML, Tortella FC. Studies on neuronal apoptosis in primary forebrain cultures: neuroprotective/anti-apoptotic action of NR2B NMDA antagonists. Neurotox Res. 2003; Jan. 5(4):255–64.10. Ahlemeyer B, Krieglstein J. Pharmacological studies supporting the therapeutic use of Ginkgo biloba extract for Alzheimer’s disease. Pharmacopsychiatry. 2003; Jun. 36 Suppl 1:S8–14.
Article11. Li S, Luo J, Wang X, Guan BC, Sun CK. Effects of Ginkgo biloba extracts on NMDA-activated currents in acutely isolated hippocampal neurons of the rat. Phytother Res. 2011; Jan. 25(1):137–41.
Article12. National Research Council of the National Academies. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press;2010.13. Sheppard A, Hayes SH, Chen GD, Ralli M, Salvi R. Review of salicylate-induced hearing loss, neurotoxicity, tinnitus and neuropathophysiology. Acta Otorhinolaryngol Ital. 2014; Apr. 34(2):79–93.14. Im GJ, Choi J, Chang JW, Kim SJ, Kim HI, Jung HH. Expression of insulin-like growth factors in a mouse model of salicylate ototoxicity. Clin Exp Otorhinolaryngol. 2010; Sep. 3(3):115–21.
Article15. Choi IS, Sung JY, Lee KS, Chang CS, Jun BH. Effects of salicylate on ABR and ECoG in guinea pig. Korean J Otolaryngol Head Neck Surg. 1999; Mar. 42(3):290–7.16. Fioretti A, Eibenstein A, Fusetti M. New trends in tinnitus management. Open Neurol J. 2011; Mar. 5:12–7.
Article17. Hwang JH, Chen JC, Yang SY, Wang MF, Liu TC, Chan YC. Expression of COX-2 and NMDA receptor genes at the cochlea and midbrain in salicylate-induced tinnitus. Laryngoscope. 2011; Feb. 121(2):361–4.18. Hu SS, Mei L, Chen JY, Huang ZW, Wu H. Expression of immediateearly genes in the dorsal cochlear nucleus in salicylate-induced tinnitus. Eur Arch Otorhinolaryngol. 2016; Feb. 273(2):325–32.
Article19. Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008; Dec. 55(7):1081–94.
Article20. Zhao J, Wang B, Wang X, Shang X. Up-regulation of Ca(2+)/CaMKII/CREB signaling in salicylate-induced tinnitus in rats. Mol Cell Biochem. 2018; Nov. 448(1-2):71–6.
Article21. Huang DS, Lin HY, Lee-Chen GJ, Hsieh-Li HM, Wu CH, Lin JY. Treatment with a Ginkgo biloba extract, EGb 761, inhibits excitotoxicity in an animal model of spinocerebellar ataxia type 17. Drug Des Devel Ther. 2016; Feb. 10:723–31.22. Wheeler D, Knapp E, Bandaru VV, Wang Y, Knorr D, Poirier C, et al. Tumor necrosis factor-alpha-induced neutral sphingomyelinase-2 modulates synaptic plasticity by controlling the membrane insertion of NMDA receptors. J Neurochem. 2009; Jun. 109(5):1237–49.23. Hwang JH, Chen JC, Chan YC. Effects of C-phycocyanin and Spirulina on salicylate-induced tinnitus, expression of NMDA receptor and inflammatory genes. PLoS One. 2013; 8(3):e58215.
Article24. Kaur S, Sharma N, Nehru B. Anti-inflammatory effects of Ginkgo biloba extract against trimethyltin-induced hippocampal neuronal injury. Inflammopharmacology. 2018; Feb. 26(1):87–104.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An Effect of Ginkgo Extract on Salicylate Ototoxicity in Guinea Pigs
- Effects of Ginkgo biloba extract on the cochlear damage induced by local gentamicin installation in guinea pigs
- The Effect of Ginkgo Biloba to Retinal Microcirculation
- Treatment Effect of Intravenous Lasix-Vitamin-Dextran and Carbogen Inhalation Therapy Accompanying Oral Drug Therapy in the Patients with Tinnitus
- The Effect of Ginkgo Biloba Extract on Diabetic Peripheral Neuropathy - A 12 week, randomized, placebo-controlled, double-blind trial -