Ann Pediatr Endocrinol Metab.
2019 Mar;24(1):60-63. 10.6065/apem.2019.24.1.60.
A case of de novo 18p deletion syndrome with panhypopituitarism
- Affiliations
-
- 1Department of Pediatrics, Inha University Hospital, Inha University College of Medicine, Incheon, Korea.
- 2Department of Pediatrics, Hanyang University Medical Center, Hanyang University School of Medicine, Seoul, Korea.
- 3Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. nadri1217@naver.com
- 4Department of Laboratory Medicine & Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2447378
- DOI: http://doi.org/10.6065/apem.2019.24.1.60
Abstract
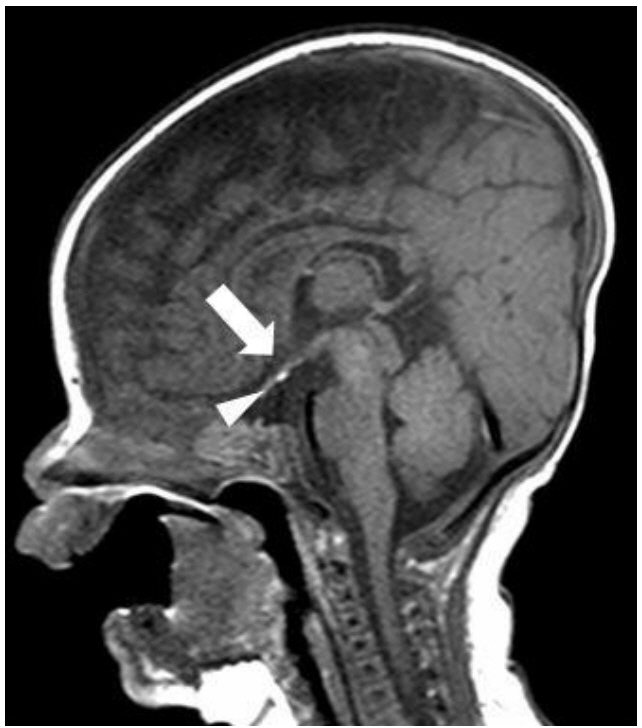
- Deletion on the short arm of chromosome 18 is a rare disorder characterized by intellectual disability, growth retardation, and craniofacial malformations (such as prominent ears, microcephaly, ptosis, and a round face). The phenotypic spectrum is wide, encompassing a range of abnormalities from minor congenital malformations to holoprosencephaly. We present a case of a 2-year-old girl with ptosis, a round face, broad neck with low posterior hairline, short stature, and panhypopituitarism. She underwent ventilation tube insertion for recurrent otitis media with effusion. Brain magnetic resonance imaging showed an ectopic posterior pituitary gland and a shallow, small sella turcica with poor visualization of the pituitary stalk. Cytogenetic and chromosomal microarray analysis revealed a de novo deletion on the short arm of chromosome 18 (arr 18p11.32p11.21[136,227-15,099,116]x1). She has been treated with recombinant human growth hormone (GH) therapy since the age of 6 months after diagnosis of GH deficiency. Her growth rate has improved without any side effects from the GH treatment. This case expands the phenotypic spectrum of 18p deletion syndrome and emphasizes the positive impact of GH therapy on linear growth in this syndrome characterized by growth deficiency. Further studies are required to define the genotype-phenotype correlation according to size and loci of the deletion in 18p deletion syndrome and to predict prognosis.
MeSH Terms
-
Arm
Brain
Child, Preschool
Chromosomes, Human, Pair 18
Cytogenetics
Diagnosis
Ear
Female
Genetic Association Studies
Growth Hormone
Holoprosencephaly
Human Growth Hormone
Humans
Intellectual Disability
Magnetic Resonance Imaging
Microarray Analysis
Microcephaly
Neck
Otitis Media with Effusion
Pituitary Gland
Pituitary Gland, Posterior
Prognosis
Sella Turcica
Ventilation
Growth Hormone
Human Growth Hormone
Figure
Reference
-
References
1. Turleau C. Monosomy 18p. Orphanet J Rare Dis. 2008; 3:4.2. Rao VB, Kerketta L, Korgaonkar S, Ghosh K, Mohanty D. 18p deletion syndrome with a 45, XY, t (14; 18) (p11;q11.2), -18, karyotype. Ann Genet. 2001; 44:187–90.
Article3. Brenk CH, Prott EC, Trost D, Hoischen A, Walldorf C, Radlwimmer B, et al. Towards mapping phenotypical traits in 18p- syndrome by array-based comparative genomic hybridisation and fluorescent in situ hybridisation. Eur J Hum Genet. 2007; 15:35–44.
Article4. Wester U, Bondeson ML, Edeby C, Annerén G. Clinical and molecular characterization of individuals with 18p deletion: a genotype-phenotype correlation. Am J Med Genet A. 2006; 140:1164–71.
Article5. Kasasbeh FA, Shawabkeh MM, Hawamdeh AA. Deletion of 18p Syndrome. Lab Med. 2011; 42:436–8.
Article6. Brandl J, Grimm T. A chromosome supplement to the London Dysmorphology Database. J Med Genet. 1987; 24:497–8.
Article7. Hasi-Zogaj M, Sebold C, Heard P, Carter E, Soileau B, Hill A, et al. A review of 18p deletions. Am J Med Genet C Semin Med Genet. 2015; 169:251–64.
Article8. de Grouchy J, Turleau C, Kusick VA. Clinical atlas of human chromosomes. 2nd ed. New York: John Wiley & Sons;1984. p. 308–13.9. Bellfield EJ, Chan J, Durrin S, Lindgren V, Shad Z, Boucher-Berry C. Anterior pituitary Aplasia in an infant with ring chromosome 18p deletion. Case Rep Endocrinol. 2016; 2016:2853178.
Article10. Artman HG, Morris CA, Stock AD. 18p- syndrome and hypopituitarism. J Med Genet. 1992; 29:671–2.11. Schaub RL, Reveles XT, Baillargeon J, Leach RJ, Cody JD. Molecular characterization of 18p deletions: evidence for a breakpoint cluster. Genet Med. 2002; 4:15–9.
Article12. Di Bella D, Lazzaro F, Brusco A, Plumari M, Battaglia G, Pastore A, et al. Mutations in the mitochondrial protease gene AFG3L2 cause dominant hereditary ataxia SCA28. Nat Genet. 2010; 42:313–21.
Article13. Glas J, Wagner J, Seiderer J, Olszak T, Wetzke M, Beigel F, et al. PTPN2 gene variants are associated with susceptibility to both Crohn's disease and ulcerative colitis supporting a common genetic disease background. PLoS One. 2012; 7:e33682.
Article14. Campbell GR, Pasquier E, Watkins J, Bourgarel-Rey V, Peyrot V, Esquieu D, et al. The glutamine-rich region of the HIV-1 Tat protein is involved in T-cell apoptosis. J Biol Chem. 2004; 279:48197–204.
Article15. Sun H, Wan N, Wang X, Chang L, Cheng D. Genotypephenotype analysis, neuropsychological assessment, and growth hormone response in a patient with 18p deletion syndrome. Cytogenet Genome Res. 2018; 154:71–8.
Article16. Rogers DG, Aswini RS. Response to growth hormone treatment in a patient with 18p-syndrome. J Pediatr Endocrinol Metab. 2012; 25:1023–5.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of De Novo 18p Deletion Syndrome with SensorineuralHearing Loss: A case report
- Patent Ductus Arteriosus and Pulmonary Valve Stenosis in A Patient with 18p Deletion Syndrome
- A Case of de Novo Interstitial Deletion 16(Q13q22)
- A Case of Partial Trisomy 2p23-pter Syndrome with Trisomy 18p Due to a de novo Supernumerary Marker Chromosome
- A Case of 18p- Syndrome