Int J Stem Cells.
2019 Mar;12(1):63-72. 10.15283/ijsc18097.
Perivascular Cells and NADPH Oxidase Inhibition Partially Restore Hyperglycemia-Induced Alterations in Hematopoietic Stem Cell and Myeloid-Derived Suppressor Cell Populations in the Bone Marrow
- Affiliations
-
- 1Department of Internal Medicine, School of Medicine, Kangwon National University, Chuncheon, Korea. shhong@kangwon.ac.kr
- 2Department of Biomedical Sciences, Stem Cell Institute, CHA University, Seongnam, Korea.
- 3Department of Molecular and Cellular Biochemistry, School of Medicine, Kangwon National University, Chuncheon, Korea.
- 4Department of Medical Environmental Biology and Tropical Medicine, School of Medicine, Kangwon National University, Chuncheon, Korea.
- 5Department of Physiology, School of Medicine, Kangwon National University, Chuncheon, Korea.
- 6Department of Hematology, Seoul St. Mary's Hospital, The Catholic University of Korea, Seoul, Korea. ckmin@catholic.ac.kr
- 7Leukemia Research Institute, The Catholic University of Korea, Seoul, Korea.
- KMID: 2447225
- DOI: http://doi.org/10.15283/ijsc18097
Abstract
- BACKGROUND AND OBJECTIVES
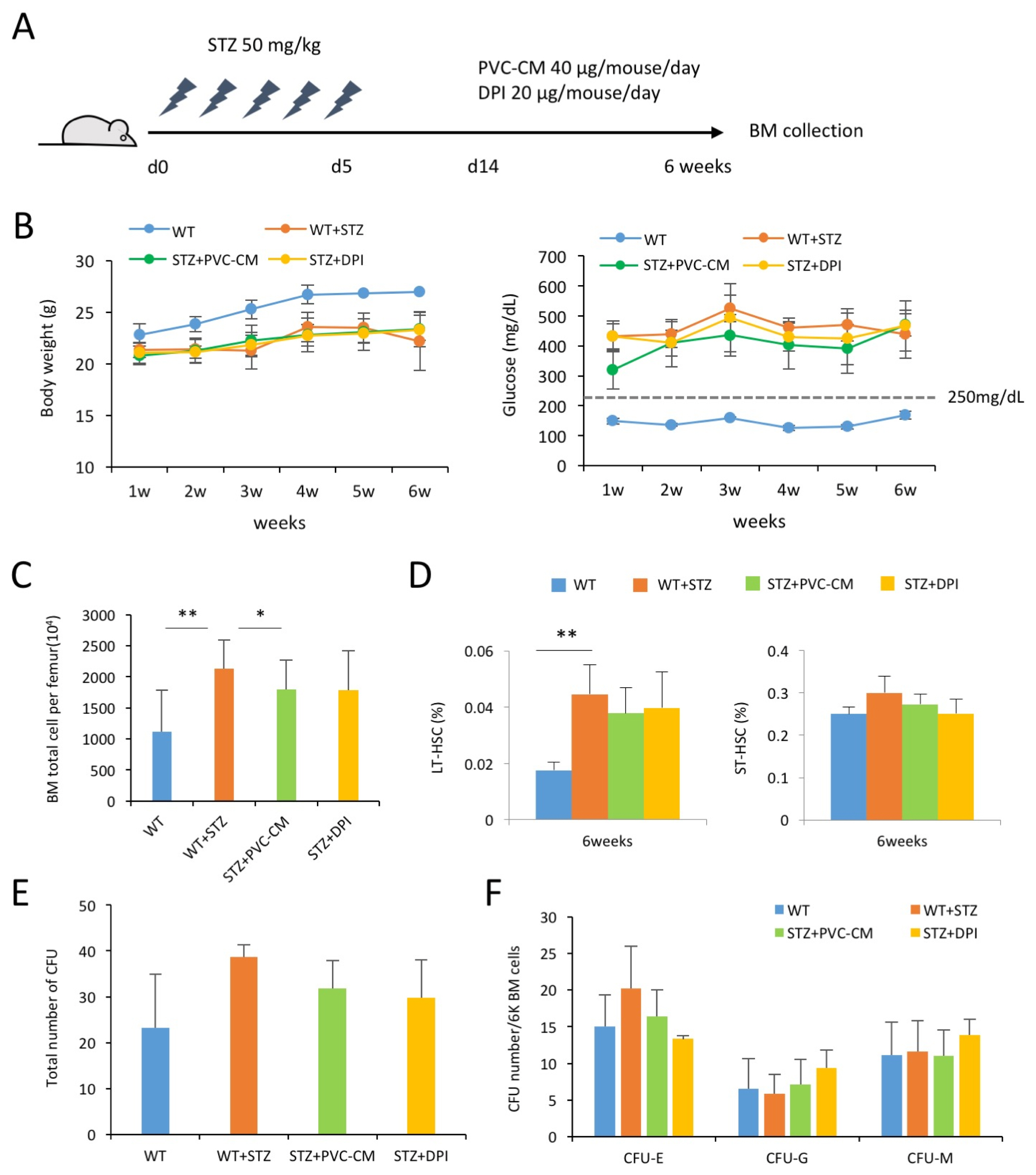
Patients suffer from long-term diabetes can result in severe complications in multiple organs through induction of vascular dysfunctions. However, the effects of chronic hyperglycemic conditions on hematopoiesis and the microenvironment in the bone marrow (BM) are not yet well understood.
METHODS
BM cells were harvested from femurs of mice and analyzed using flow cytometry. Human PVCs were cultured in serum-free α-MEM. After 24hrs, PVC-CM was collected and filtered through a 0.22 μm filter.
RESULTS
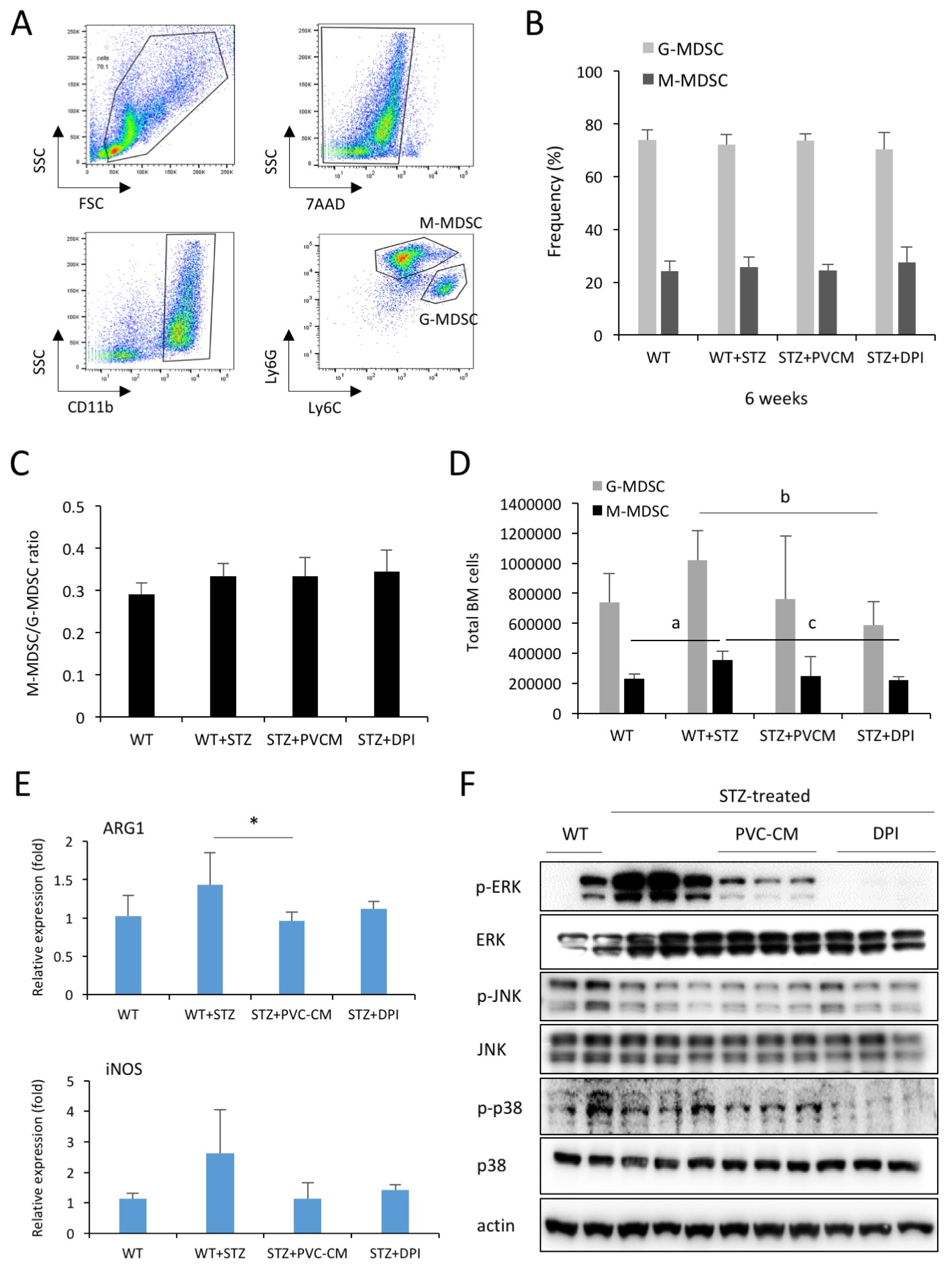
In this study, we showed that hyperglycemia alters hematopoietic composition in the BM, which can partially be restored via paracrine mechanisms, including perivascular cells (PVCs) and NADPH oxidase (NOX) inhibition in mice with streptozotocin-induced diabetes. Prolonged hyperglycemic conditions resulted in an increase in the frequency and number of long-term hematopoietic stem cells as well as the number of total BM cells. The altered hematopoiesis in the BM was partially recovered by treatment with PVC-derived conditioned medium (CM). Long-term diabetes also increased the number of myeloid-derived suppressor cells in the BM, which was partially restored by the administration of PVC-CM and diphenyleneiodonium (DPI), a NOX inhibitor. We further showed the downregulation of ERK and p38 phosphorylation in BM cells of diabetic mice treated with PVC-CM and DPI. This may be associated with dysfunction of hematopoietic cells and promotion of subsequent diabetic complications.
CONCLUSIONS
Our data suggested that alterations in BM hematopoietic composition due to prolonged hyperglycemic conditions might be restored by improvement of the hematopoietic microenvironment and modulation of NOX activity.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Hematopoietic Stem Cells and Their Roles in Tissue Regeneration
Ji Yoon Lee, Seok-Ho Hong
Int J Stem Cells. 2019;13(1):1-12. doi: 10.15283/ijsc19127.Perivascular Stem Cells Suppress Inflammasome Activation during Inflammatory Responses in Macrophages
Jeeyoung Kim, Woo Jin Kim, Kwon-Soo Ha, Eun-Taek Han, Won Sun Park, Se-Ran Yang, Seok-Ho Hong
Int J Stem Cells. 2019;12(3):419-429. doi: 10.15283/ijsc19115.
Reference
-
References
1. Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world--a growing challenge. N Engl J Med. 2007; 356:213–215. DOI: 10.1056/NEJMp068177. PMID: 17229948.
Article2. Nagareddy PR, Murphy AJ, Stirzaker RA, Hu Y, Yu S, Miller RG, Ramkhelawon B, Distel E, Westerterp M, Huang LS, Schmidt AM, Orchard TJ, Fisher EA, Tall AR, Goldberg IJ. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 2013; 17:695–708. DOI: 10.1016/j.cmet.2013.04.001. PMID: 23663738. PMCID: 3992275.
Article3. Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonneveld AJ. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004; 53:195–199. DOI: 10.2337/diabetes.53.1.195.4. Fadini GP, Sartore S, Albiero M, Baesso I, Murphy E, Menegolo M, Grego F, Vigili de Kreutzenberg S, Tiengo A, Agostini C, Avogaro A. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vasc Biol. 2006; 26:2140–2146. DOI: 10.1161/01.ATV.0000237750.44469.88. PMID: 16857948.
Article5. Ferraro F, Lymperi S, Méndez-Ferrer S, Saez B, Spencer JA, Yeap BY, Masselli E, Graiani G, Prezioso L, Rizzini EL, Mangoni M, Rizzoli V, Sykes SM, Lin CP, Frenette PS, Quaini F, Scadden DT. Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Sci Transl Med. 2011; 3:104ra101. DOI: 10.1126/scitranslmed.3002191. PMID: 21998408. PMCID: 3754876.
Article6. Fadini GP, Fiala M, Cappellari R, Danna M, Park S, Poncina N, Menegazzo L, Albiero M, DiPersio J, Stockerl-Goldstein K, Avogaro A. Diabetes limits stem cell mobilization following G-CSF but not plerixafor. Diabetes. 2015; 64:2969–2977. DOI: 10.2337/db15-0077. PMID: 25804941. PMCID: 4512229.
Article7. Han X, Deng Y, Yu J, Sun Y, Ren G, Cai J, Zhu J, Jiang G. Acarbose accelerates wound healing via Akt/eNOS signaling in db/db mice. Oxid Med Cell Longev. 2017; 2017:7809581. DOI: 10.1155/2017/7809581. PMID: 28373902. PMCID: 5360971.8. Kojima H, Kim J, Chan L. Emerging roles of hematopoietic cells in the pathobiology of diabetic complications. Trends Endocrinol Metab. 2014; 25:178–187. DOI: 10.1016/j.tem.2014.01.002. PMID: 24507996. PMCID: 3975817.
Article9. Kim H, Han JW, Lee JY, Choi YJ, Sohn YD, Song M, Yoon YS. Diabetic mesenchymal stem cells are ineffective for improving limb ischemia due to their impaired angiogenic capability. Cell Transplant. 2015; 24:1571–1584. DOI: 10.3727/096368914X682792. PMID: 25008576. PMCID: 4621803.
Article10. Holmes D. Diabetes: SDF-1 dysregulation mediates diabetic stem cell mobilopathy. Nat Rev Endocrinol. 2015; 11:318. DOI: 10.1038/nrendo.2015.63. PMID: 25869572.
Article11. Morohoshi M, Fujisawa K, Uchimura I, Numano F. Glucose-dependent interleukin 6 and tumor necrosis factor production by human peripheral blood monocytes in vitro. Diabetes. 1996; 45:954–959. DOI: 10.2337/diab.45.7.954. PMID: 8666148.
Article12. Guha M, Bai W, Nadler JL, Natarajan R. Molecular mechanisms of tumor necrosis factor alpha gene expression in monocytic cells via hyperglycemia-induced oxidant stressdependent and -independent pathways. J Biol Chem. 2000; 275:17728–17739. DOI: 10.1074/jbc.275.23.17728. PMID: 10837498.
Article13. Kim H, Cho HJ, Kim SW, Liu B, Choi YJ, Lee J, Sohn YD, Lee MY, Houge MA, Yoon YS. CD31+ cells represent highly angiogenic and vasculogenic cells in bone marrow: novel role of nonendothelial CD31+ cells in neovascularization and their therapeutic effects on ischemic vascular disease. Circ Res. 2010; 107:602–614. DOI: 10.1161/CIRCRESAHA.110.218396. PMID: 20634489. PMCID: 2938961.
Article14. Wang T, Wen Y, Fan X. Myeloid-derived suppressor cells suppress CD4+ T cell activity and prevent the development of type 2 diabetes. Acta Biochim Biophys Sin (Shanghai). 2018; 50:362–369. DOI: 10.1093/abbs/gmy014. PMID: 29514172.
Article15. Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008; 111:4233–4244. DOI: 10.1182/blood-2007-07-099226. PMID: 18272812.
Article16. Zhou Z, French DL, Ma G, Eisenstein S, Chen Y, Divino CM, Keller G, Chen SH, Pan PY. Development and function of myeloid-derived suppressor cells generated from mouse embryonic and hematopoietic stem cells. Stem Cells. 2010; 28:620–632. PMID: 20073041. PMCID: 4370270.
Article17. Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009; 9:162–174. DOI: 10.1038/nri2506. PMID: 19197294. PMCID: 2828349.
Article18. Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005; 5:641–654. DOI: 10.1038/nri1668. PMID: 16056256.
Article19. Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978; 4:7–25. PMID: 747780.20. Mercier FE, Ragu C, Scadden DT. The bone marrow at the crossroads of blood and immunity. Nat Rev Immunol. 2011; 12:49–60. DOI: 10.1038/nri3132. PMID: 22193770. PMCID: 4013788.
Article21. Rafii S, Mohle R, Shapiro F, Frey BM, Moore MA. Regulation of hematopoiesis by microvascular endothelium. Leuk Lymphoma. 1997; 27:375–386. DOI: 10.3109/10428199709058305. PMID: 9477120.
Article22. Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007; 131:324–336. DOI: 10.1016/j.cell.2007.08.025. PMID: 17956733.
Article23. Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006; 25:977–988. DOI: 10.1016/j.immuni.2006.10.016. PMID: 17174120.
Article24. Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012; 481:457–462. DOI: 10.1038/nature10783. PMID: 22281595. PMCID: 3270376.
Article25. Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K, Mar JC, Bergman A, Frenette PS. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013; 502:637–643. DOI: 10.1038/nature12612. PMID: 24107994. PMCID: 3821873.
Article26. Corselli M, Chin CJ, Parekh C, Sahaghian A, Wang W, Ge S, Evseenko D, Wang X, Montelatici E, Lazzari L, Crooks GM, Péault B. Perivascular support of human hematopoietic stem/progenitor cells. Blood. 2013; 121:2891–2901. DOI: 10.1182/blood-2012-08-451864. PMID: 23412095. PMCID: 3707421.
Article27. Levesque JP. A niche in a dish: pericytes support HSC. Blood. 2013; 121:2816–2818. DOI: 10.1182/blood-2013-02-485144. PMID: 23580633.
Article28. Hammes HP, Lin J, Renner O, Shani M, Lundqvist A, Betsholtz C, Brownlee M, Deutsch U. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes. 2002; 51:3107–3112. DOI: 10.2337/diabetes.51.10.3107. PMID: 12351455.
Article29. Warmke N, Griffin KJ, Cubbon RM. Pericytes in diabetesassociated vascular disease. J Diabetes Complications. 2016; 30:1643–1650. DOI: 10.1016/j.jdiacomp.2016.08.005. PMID: 27592245.
Article30. An B, Kim E, Song H, Ha KS, Han ET, Park WS, Ahn TG, Yang SR, Na S, Hong SH. Gestational diabetes affects the growth and functions of perivascular stem cells. Mol Cells. 2017; 40:434–439. DOI: 10.14348/molcells.2017.0053. PMID: 28614916. PMCID: 5523020.
Article31. Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012; 10:244–258. DOI: 10.1016/j.stem.2012.02.005. PMID: 22385653. PMCID: 3294273.
Article32. Whitfield-Larry F, Felton J, Buse J, Su MA. Myeloid-derived suppressor cells are increased in frequency but not maximally suppressive in peripheral blood of Type 1 Diabetes Mellitus patients. Clin Immunol. 2014; 153:156–164. DOI: 10.1016/j.clim.2014.04.006. PMID: 24769355.
Article33. Hassan M, Raslan HM, Eldin HG, Mahmoud E, Elwajed HAA. CD33+ HLA-DR-myeloid-derived suppressor cells are increased in frequency in the peripheral blood of Type1 diabetes patients with predominance of CD14+ subset. Open Access Maced J Med Sci. 2018; 6:303–309. DOI: 10.3889/oamjms.2018.080. PMID: 29531593. PMCID: 5839437.
Article34. Zhang W, Thompson BJ, Hietakangas V, Cohen SM. MAPK/ERK signaling regulates insulin sensitivity to control glucose metabolism in Drosophila. PLoS Genet. 2011; 7:e1002429. DOI: 10.1371/journal.pgen.1002429. PMID: 22242005. PMCID: 3248469.
Article35. Igarashi M, Wakasaki H, Takahara N, Ishii H, Jiang ZY, Yamauchi T, Kuboki K, Meier M, Rhodes CJ, King GL. Glucose or diabetes activates p38 mitogen-activated protein kinase via different pathways. J Clin Invest. 1999; 103:185–195. DOI: 10.1172/JCI3326. PMID: 9916130. PMCID: 407875.
Article36. Bennett BL, Satoh Y, Lewis AJ. JNK: a new therapeutic target for diabetes. Curr Opin Pharmacol. 2003; 3:420–425. DOI: 10.1016/S1471-4892(03)00068-7. PMID: 12901952.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Double-unit Cord Blood Transplantation in Primary Refractory Acute Myeloid Leukemia
- Analysis of the Expression and Regulation of PD-1 Protein on the Surface of Myeloid-Derived Suppressor Cells (MDSCs)
- 5-aminoimidazole-4-carboxamide Riboside Induces Apoptosis Through AMP-activated Protein Kinase-independent and NADPH Oxidase-dependent Pathways
- Bone marrow-derived stem cells contribute to regeneration of the endometrium
- Apoptosis: role in myeloid cell development