Yonsei Med J.
2019 Jun;60(6):525-534. 10.3349/ymj.2019.60.6.525.
Real-World Analysis of the Efficacy of Rebiopsy and EGFR Mutation Test of Tissue and Plasma Samples in Drug-Resistant Non-Small Cell Lung Cancer
- Affiliations
-
- 1Division of Medical Oncology, Department of Internal Medicine, Yonsei Cancer Center, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. cbc1971@yuhs.ac
- KMID: 2446957
- DOI: http://doi.org/10.3349/ymj.2019.60.6.525
Abstract
- PURPOSE
Standard treatment for cases of non-small cell lung cancer (NSCLC) exhibiting acquired drug resistance includes tumor rebiopsy, epidermal growth factor receptor (EGFR) mutation testing (e.g., for T790M mutations), and the subsequent administration of third-generation EGFR-tyrosine kinase inhibitors (EGFR-TKIs). However, rebiopsies are typically invasive, costly, and occasionally not feasible. Therefore, the present study aimed to assess rebiopsy procedures by analyzing real-world data collected by the ASTRIS study of patients with resistant NSCLC.
MATERIALS AND METHODS
The present study used statistical models to evaluate data collected by the ASTRIS trial (NCT02474355) conducted at Yonsei Cancer Center, including the rebiopsy success rate, incidence of T790M mutations in collected tissue and plasma samples, and association of administered osimertinib treatment efficacy.
RESULTS
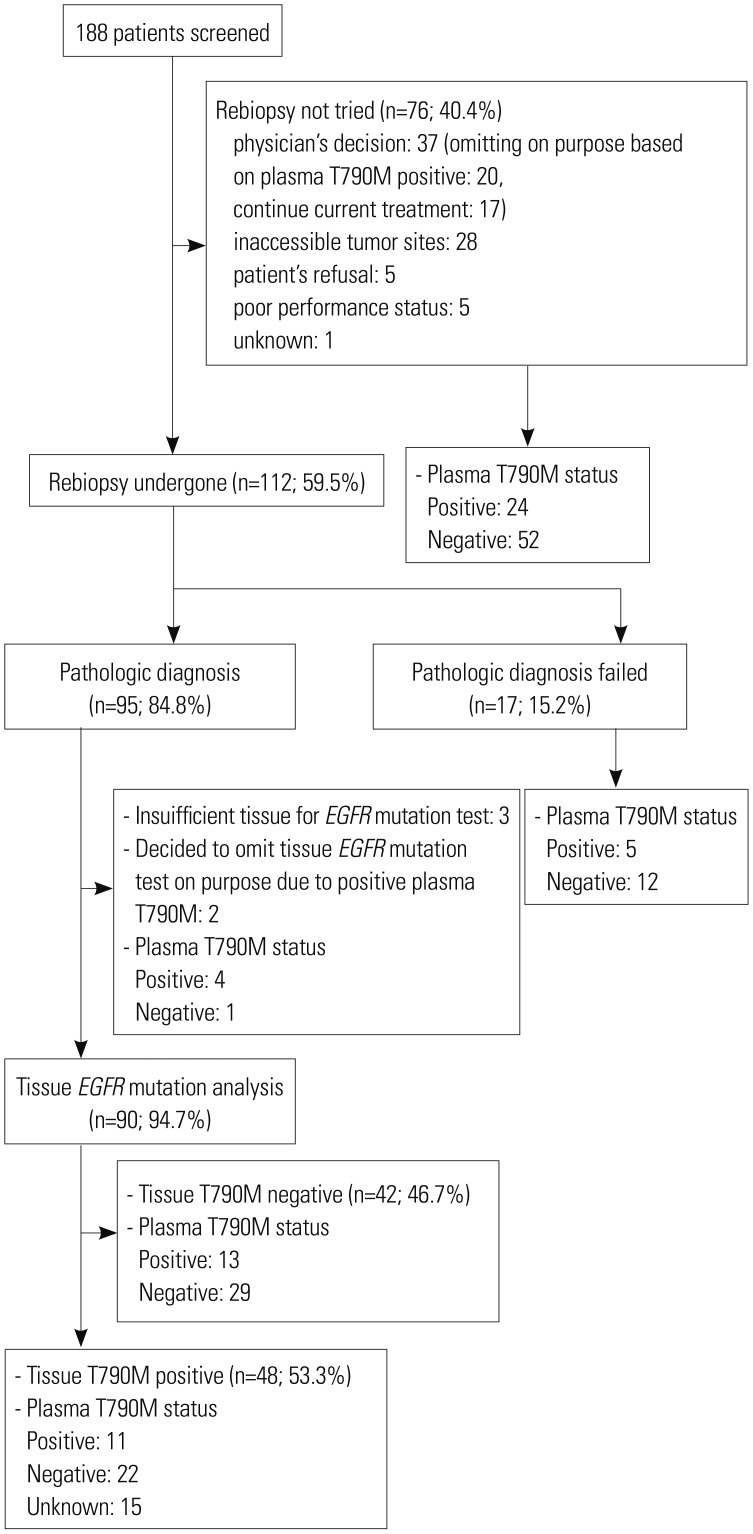
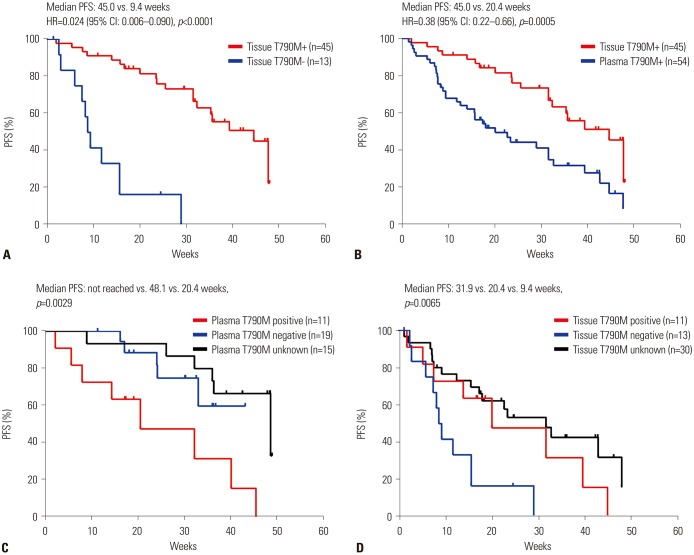
In a total of 188 screened patients, 112 underwent rebiopsy. An adequate tumor specimen was obtained in 95 of these patients, the greatest majority of whom (43.8%) were subjected to bronchoscopy. T790M mutations were detected in 53.3% of successfully EGFR-tested rebiopsy samples. A total of 88 patients received osimertinib treatment, and the objective response rate and median progression-free survival time was 44.3% and 32.7 weeks, respectively, among the treated patients overall, but 57.8% and 45.0 weeks, and 35.2% and 20.4 weeks among patients who exhibited T790M-positive tissue (n=45) and plasma (n=54) samples, respectively.
CONCLUSION
Approximately 60% of patients in the analyzed real-world cohort were eligible for tissue rebiopsy upon NSCLC progression. Osimertinib activity was higher in patients in whom T790M mutations were detected in tissues rather than in plasma samples.
MeSH Terms
Figure
Reference
-
1. Riely GJ, Yu HA. EGFR: the paradigm of an oncogene-driven lung cancer. Clin Cancer Res. 2015; 21:2221–2226. PMID: 25979928.
Article2. Costanzo R, Montanino A, Di Maio M, Piccirillo MC, Sandomenico C, Giordano P, et al. Advanced non-small-cell lung cancer with epidermal growth factor receptor mutations: current evidence and future perspectives. Expert Rev Anticancer Ther. 2013; 13:1207–1218. PMID: 24134422.
Article3. Melosky B. Review of EGFR TKIs in metastatic NSCLC, including ongoing trials. Front Oncol. 2014; 4:244. PMID: 25309870.4. Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat Rev Clin Oncol. 2014; 11:473–481. PMID: 24981256.
Article5. Westover D, Zugazagoitia J, Cho BC, Lovly CM, Paz-Ares L. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol. 2018; 29(suppl_1):i10–i19. PMID: 29462254.
Article6. Lim SM, Syn NL, Cho BC, Soo RA. Acquired resistance to EGFR targeted therapy in non-small cell lung cancer: mechanisms and therapeutic strategies. Cancer Treat Rev. 2018; 65:1–10. PMID: 29477930.
Article8. Mok TS, Wu Y-L, Ahn M-J, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017; 376:629–640. PMID: 27959700.
Article9. Liang Z, Cheng Y, Chen Y, Hu Y, Liu WP, Lu Y, et al. EGFR T790M ctDNA testing platforms and their role as companion diagnostics: correlation with clinical outcomes to EGFR-TKIs. Cancer Lett. 2017; 403:186–194. PMID: 28642172.
Article10. Kawamura T, Kenmotsu H, Taira T, Omori S, Nakashima K, Wakuda K, et al. Rebiopsy for patients with non-small-cell lung cancer after epidermal growth factor receptor-tyrosine kinase inhibitor failure. Cancer Sci. 2016; 107:1001–1005. PMID: 27145431.
Article11. Reckamp KL, Melnikova VO, Karlovich C, Sequist LV, Camidge DR, Wakelee H, et al. A highly sensitive and quantitative test platform for detection of NSCLC EGFR mutations in urine and plasma. J Thorac Oncol. 2016; 11:1690–1700. PMID: 27468937.
Article12. Bernabé R, Hickson N, Wallace A, Blackhall FH. What do we need to make circulating tumour DNA (ctDNA) a routine diagnostic test in lung cancer? Eur J Cancer. 2017; 81:66–73. PMID: 28609695.
Article13. Novello S, Barlesi F, Califano R, Cufer T, Ekman S, Levra MG, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016; 27(suppl 5):v1–v27. PMID: 27664245.
Article14. De Marinis F, Cho BC, Kim DW, Kim SW, Hochmair MJ, Metro G, et al. ASTRIS: a real world treatment study of osimertinib in patients (pts) with EGFR T790M positive non-small cell lung cancer (NSCLC). J Clin Oncol. 2017; 35(5_suppl):15.
Article15. Kim HJ, Lee KY, Kim YC, Kim KS, Lee SY, Jang TW, et al. Detection and comparison of peptide nucleic acid-mediated real-time polymerase chain reaction clamping and direct gene sequencing for epidermal growth factor receptor mutations in patients with non-small cell lung cancer. Lung Cancer. 2012; 75:321–325. PMID: 21930325.
Article16. Han JY, Choi JJ, Kim JY, Han YL, Lee GK. PNA clamping-assisted fluorescence melting curve analysis for detecting EGFR and KRAS mutations in the circulating tumor DNA of patients with advanced non-small cell lung cancer. BMC Cancer. 2016; 16:627. PMID: 27519791.
Article17. Paz-Ares L, Tan EH, O'Byrne K, Zhang L, Hirsh V, Boyer M, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol. 2017; 28:270–277. PMID: 28426106.
Article18. Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015; 16:141–151. PMID: 25589191.
Article19. Lee CK, Davies L, Wu YL, Mitsudomi T, Inoue A, Rosell R, et al. Gefitinib or erlotinib vs chemotherapy for EGFR mutation-positive lung cancer: individual patient data meta-analysis of overall survival. J Natl Cancer Inst. 2017; 109:djw279.
Article20. Chouaid C, Dujon C, Do P, Monnet I, Madroszyk A, Le Caer H, et al. Feasibility and clinical impact of re-biopsy in advanced non small-cell lung cancer: a prospective multicenter study in a real-world setting (GFPC study 12-01). Lung Cancer. 2014; 86:170–173. PMID: 25214431.
Article21. Hasegawa T, Sawa T, Futamura Y, Horiba A, Ishiguro T, Marui T, et al. Feasibility of rebiopsy in non-small cell lung cancer treated with epidermal growth factor receptor-tyrosine kinase inhibitors. Intern Med. 2015; 54:1977–1980. PMID: 26278287.
Article22. Nosaki K, Satouchi M, Kurata T, Yoshida T, Okamoto I, Katakami N, et al. Re-biopsy status among non-small cell lung cancer patients in Japan: a retrospective study. Lung Cancer. 2016; 101:1–8. PMID: 27794396.
Article23. Sacher AG, Komatsubara KM, Oxnard GR. Application of plasma genotyping technologies in non-small cell lung cancer: a practical review. J Thorac Oncol. 2017; 12:1344–1356. PMID: 28611011.
Article24. Moding EJ, Diehn M, Wakelee HA. Circulating tumor DNA testing in advanced non-small cell lung cancer. Lung Cancer. 2018; 119:42–47. PMID: 29656751.
Article25. Kawamura T, Kenmotsu H, Omori S, Nakashima K, Wakuda K, Ono A, et al. Clinical factors predicting detection of T790M mutation in rebiopsy for EGFR-mutant non-small-cell lung cancer. Clin Lung Cancer. 2018; 19:e247–e252. PMID: 28866043.
Article26. Jenkins S, Yang JC, Ramalingam SS, Yu K, Patel S, Weston S, et al. Plasma ctDNA analysis for detection of the EGFR T790M mutation in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2017; 12:1061–1070. PMID: 28428148.27. Kim CG, Shim HS, Hong MH, Cha YJ, Heo SJ, Park HS, et al. Detection of activating and acquired resistant mutation in plasma from EGFR-mutated NSCLC patients by peptide nucleic acid (PNA) clamping-assisted fluorescence melting curve analysis. Oncotarget. 2017; 8:65111–65122. PMID: 29029416.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Should We Perform Repeated Re-biopsy for the Detection of T790M Mutation?
- Detection of EGFR and KRAS Mutation by Pyrosequencing Analysis in Cytologic Samples of Non-Small Cell Lung Cancer
- EGFR C797S as a Resistance Mechanism of Lazertinib in Non-small Cell Lung Cancer with EGFR T790M Mutation
- Cost-Effectiveness Analysis of Three Diagnostic Strategies for the Detection of EGFR Mutation in Advanced Non-Small Cell Lung Cancer
- Small Cell Lung Cancer with Mutation of Epidermal Growth Factor Receptor in Patients with Lung Adenocarcinoma Resistant to Gefitinib