Neurointervention.
2019 Mar;14(1):1-8. 10.5469/neuroint.2019.00031.
Protection of Personal Information in Medical Journal Publications
- Affiliations
-
- 1Department of Parasitology and Institute of Medical Education, Hallym University College of Medicine, Chuncheon, Korea. shuh@hallym.ac.kr
- KMID: 2446776
- DOI: http://doi.org/10.5469/neuroint.2019.00031
Abstract
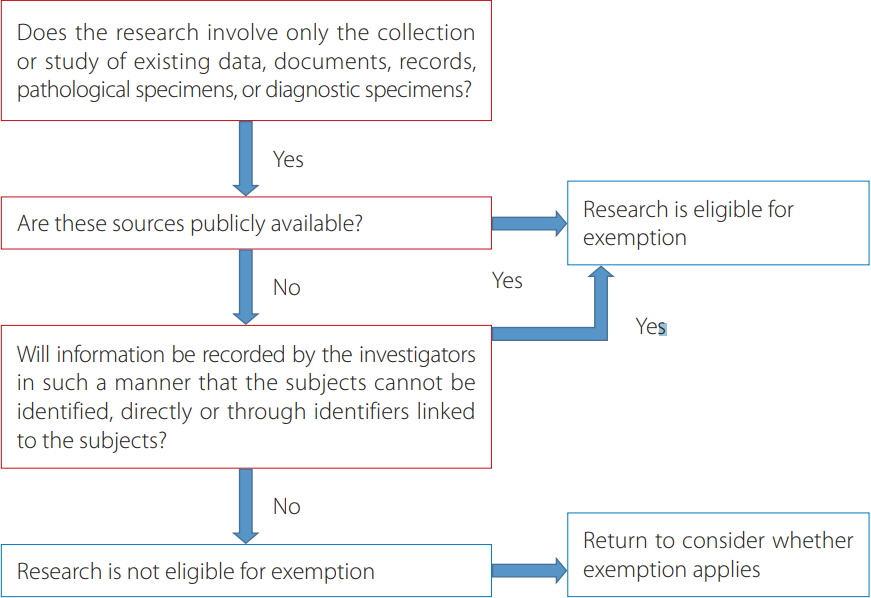
- It aimed to present the definition of personal information based on Korean laws that protect personal information and the process of protection of personal information in journal publishing based on the guidelines of the International Committee of Medical Journal Editors and Committee of Publication Ethics. Two Korean laws relate to the protection of personal information in human subject research: the Personal Information Protection Act and the Bioethics and Safety Act. These laws were enacted to prevent the unauthorized use of Koreans' personal information including medical information. Personal information can be divided into personally identifiable information including resident registration numbers and sensitive information including health information. To protect personal information in journal publishing, institutional review board (IRB) approval and obtaining informed consent from patients is recommended or mandatory in clinical studies. However, retrospective chart reviews may be exempted from IRB approval, while obtaining informed consent is recommended for all case reports. Journal policies may vary with regard to whether a copy of the informed consent form is collected from authors, since the Committee of Publication Ethics guideline does not specifically recommend collecting it. In discussions of adopting clinical data-sharing policies, transfer of data including nonidentifiable personal information to another country is an unresolved issue. Furthermore, a public data repository site should be established in Korea for data to be deposited. To protect subjects' privacy and to prevent legal issues potentially arising from privacy concerns, editors and publishers should do their best to publish articles with appropriate oversight on subjects' personal information.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Data Pseudonymization in a Range That Does Not Affect Data Quality: Correlation with the Degree of Participation of Clinicians
Soo-Yong Shin, Hun-Sung Kim
J Korean Med Sci. 2021;36(44):e299. doi: 10.3346/jkms.2021.36.e299.
Reference
-
1. Ministry of Government Legislation of Korea. Personal information protection act. http://elaw.klri.re.kr/kor_service/lawView.do?lang=ENG&hseq=46731. Accessed January 19, 2019.2. Ministry of Government Legislation of Korea. Bioethics and safety. http://elaw.klri.re.kr/kor_service/lawView.do?hseq=46341&lang=ENG. Accessed January 19, 2019.3. International Committee of Medical Journal Editors. Protection of research participants. http://www.icmje.org/recommendations/browse/roles-and-responsibilities/protection-of-research-participants.html. Accessed January 20, 2019.4. Committee of Publication Ethics. Journals’ best practice for ensuring consent for publishing medical case reports: guidance for COPE. https://publicationethics.org/resources/guidelines/journals%E2%80%99-best-practices-ensuring-consent-publishing-medical-case-reports. Accessed December 31, 2018.5. Ministry of Government Legislation of Korea. Enforcement decree of the personal information protection act. http://elaw.klri.re.kr/kor_service/lawView.do?lang=ENG&hseq=45683. Accessed January 20, 2019.6. Ministry of Government Legislation of Korea. Enforcement rule of bioethics and safety act. http://www.law.go.kr/LSW/lsInfoP.do?lsiSeq=206257#0000. Accessed January 20, 2019.7. Korean Society of Interventional Neuroradiology. Research and publication ethics. https://neurointervention.org/authors/ethics.php. Accessed December 31, 2018.8. Office of Human Research Protections. US Department of Health & Human Services. Human subject regulations decision charts. https://www.hhs.gov/ohrp/regulations-and-policy/decision-charts/index.html. Accessed December 31, 2018.9. Huh S. Strengthened research ethics, including patient anonymity and informed consent, in MEDLINE and PubMed Central journals. Arch Craniofac Surg. 2018; 19:241–242.
Article10. Editorial Office, Archives of Plastic Surgery. Corrigenda: omission of the description of informed consent on the identifiable photos and the description on ethical treatment of experimental animals. Arch Plast Surg. 2017; 44:575–576.
Article11. Huh S. How to deal with ethical issues involving animal experiments and identifiable photographs in articles published in Archives of Plastic Surgery. Arch Plast Surg. 2017; 44:475–476.
Article12. Huh S. Adherence of the Annals of Pediatric Endocrinology and Metabolism to the Principles of Transparency and Best Practice in scholarly publishing. Ann Pediatr Endocrinol Metab. 2018; 23:1–3.13. Huh S. How to add a journal to the international databases, Science Citation Index Expanded and MEDLINE. Arch Plast Surg. 2016; 43:487–490.
Article14. Huh S. How to prepare Endocrinology and Metabolism for reapplication to MEDLINE. Endocrinol Metab (Seoul). 2017; 32:58–61.15. Huh S. Ethical issue in preparing manuscript on esthetic patients. Arch Aesthetic Plast Surg. 2014; 20:1–2.
Article16. Huh S. Updates from 2018: being indexed in EMBASE, becoming an affiliated journal of the World Federation for Medical Education, implementing an optional open data policy, adopting principles of transparency and best practice in scholarly publishing, and appreciation to reviewers. J Educ Eval Health Prof. 2018; 15:36.
Article17. Mello MM, Lieou V, Goodman SN. Clinical trial participants’ views of the risks and benefits of data sharing. N Engl J Med. 2018; 378:2202–2211.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Factors Influencing Clinical Nurses' Practice of Personal Information Protection: Focusing on Knowledge of Personal Information Protection Law and Nursing Patient Advocacy
- Using medical big data for clinical research and legal considerations for the protection of personal information: the double-edged sword
- Knowledge and Practice of Dental Practitioners Regarding Patient's Personal Information
- Factors Associated with Practice of Health Information Protection among Nursing Students
- Self-Quarantine System and Personal Information Privacy in South Korea