J Nutr Health.
2019 Apr;52(2):168-175. 10.4163/jnh.2019.52.2.168.
Anti-melanogenic effects of Hordeum vulgare L. barely sprout extract in murine B16F10 melanoma cells
- Affiliations
-
- 1Department of Food Science and Nutrition, Keimyung University, Daegu 42601, Korea.
- 2Research Development Team, Food Industry Research Center, Jeonnam Bioindustry Foundation, Naju, Jeonnam 58275, Korea. methyl@nate.com
- KMID: 2444940
- DOI: http://doi.org/10.4163/jnh.2019.52.2.168
Abstract
- PURPOSE
Barely sprout is a well-known oriental herbal medicine with a wide range of health benefits. Recent studies have provided scientific evidence of its therapeutic effects with expanded application. This study investigated anti-melanogenic effect of barley sprout water extract (BSE) in murine melanocyte B16F10.
METHODS
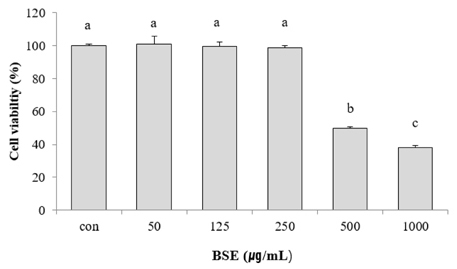
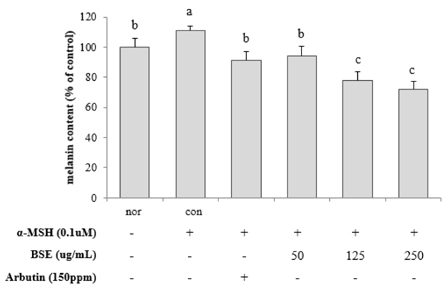
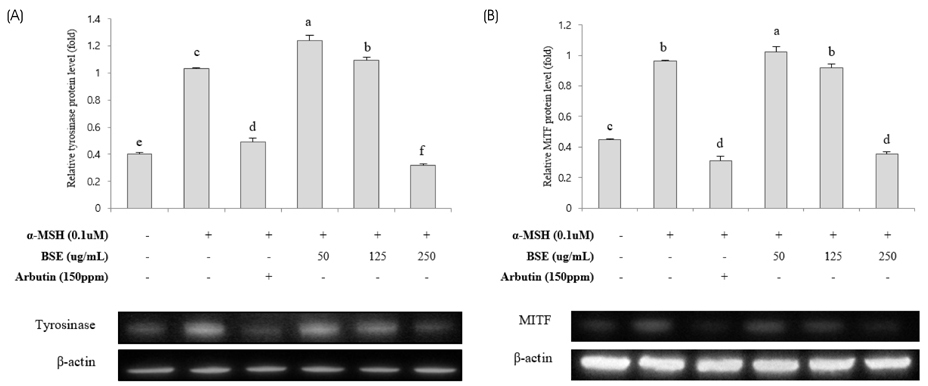
Various concentrations (0, 50, 125, and 250 µg/mL) of BSE and arbutin (150 ppm) were applied to B16F10 stimulated with or without alpha-melanocyte stimulating hormone (100 nM) for 72 hours. The whitening potency of BSE was determined altered cellular melanin contents. Activity and expression of tyrosinase and microphthalmia-associated transcription factor (MITF) were also assayed.
RESULTS
Experimental results revealed that treatment with BSE reduced cellular melanin production by approximately 40% compared to the control. Molecular findings supported that suppressed activity and expression of tyrosinase and MITF proteins by BSE were associated with declined cellular melanogenesis. Furthermore, anti-melanogenic effect of BSE (250 µg/mL) was similar to that of arbutin, a commonly used whitening agent. Lastly, polyphenols including p-coumaric, ferulic, and vanillic acids were identified in BSE using HPLC analyses. They might be potential active ingredients showing such melanogenesis-reducing effect.
CONCLUSION
BSE was evident to possess favorable anti-melanogenic potency in an in vitro model. As a natural food sourced material, BSE could be an effective depigmentation agent with potential application in pharmaceutical and cosmetic industries.
Keyword
MeSH Terms
-
Arbutin
Chromatography, High Pressure Liquid
Herbal Medicine
Hordeum*
In Vitro Techniques
Insurance Benefits
Melanins
Melanocytes
Melanoma*
Microphthalmia-Associated Transcription Factor
Monophenol Monooxygenase
Polyphenols
Therapeutic Uses
Vanillic Acid
Water
Arbutin
Melanins
Microphthalmia-Associated Transcription Factor
Monophenol Monooxygenase
Polyphenols
Therapeutic Uses
Vanillic Acid
Water
Figure
Reference
-
1. Lin JW, Chiang HM, Lin YC, Wen KC. Natural products with skin-whitening effects. J Food Drug Anal. 2008; 16(2):1–10.2. Pillaiyar T, Manickam M, Jung SH. Recent development of signaling pathways inhibitors of melanogenesis. Cell Signal. 2017; 40:99–115.
Article3. Liu-Smith F, Meyskens FL. Molecular mechanisms of flavonoids in melanin synthesis and the potential for the prevention and treatment of melanoma. Mol Nutr Food Res. 2016; 60(6):1264–1274.
Article4. Shim E, Song E, Choi KS, Choi HJ, Hwang J. Inhibitory effect of Gastrodia elata Blume extract on alpha-melanocyte stimulating hormone-induced melanogenesis in murine B16F10 melanoma. Nutr Res Pract. 2017; 11(3):173–179.
Article5. Chen WC, Tseng TS, Hsiao NW, Lin YL, Wen ZH, Tsai CC, et al. Discovery of highly potent tyrosinase inhibitor, T1, with significant anti-melanogenesis ability by zebrafish in vivo assay and computational molecular modeling. Sci Rep. 2015; 5(1):7995.
Article6. Shin BY, Jung BR, Jung JG, Cho SA, Bang MA. Inhibitory effects on melanin production in B16 melanoma cells of fallen pear. J Korean Soc Food Sci Nutr. 2017; 46(3):320–326.
Article7. Arndt KA, Fitzpatrick TB. Topical use of hydroquinone as a depigmenting agent. JAMA. 1965; 194(9):965–967.
Article8. Funayama M, Arakawa H, Yamamoto R, Nishino T, Shin T, Murao S. Effects of alpha- and beta-arbutin on activity of tyrosinases from mushroom and mouse melanoma. Biosci Biotechnol Biochem. 1995; 59(1):143–144.9. Ros JR, Rodríguez-López JN, García-Cánovas F. Effect of L-ascorbic acid on the monophenolase activity of tyrosinase. Biochem J. 1993; 295(Pt 1):309–312.
Article10. Mishima Y, Hatta S, Ohyama Y, Inazu M. Induction of melanogenesis suppression: cellular pharmacology and mode of differential action. Pigment Cell Res. 1988; 1(6):367–374.
Article11. Chang TS. Natural melanogenesis inhibitors acting through the down-regulation of tyrosinase activity. Materials (Basel). 2012; 5(9):1661–1685.
Article12. Fuyuno I. Spotlight turns on cosmetics for Asian skin. Nature. 2004; 432(7020):938.
Article13. Elhadi Aborus N, Čanadanović-Brunet J, Ćetković G, Tumbas Šaponjac V, Vulić J, Ilić N. Powdered barley sprouts: composition, functionality and polyphenol digestibility. Int J Food Sci Technol. 2016; 52(1):231–238.
Article14. Lahouar L, El-Bok S, Achour L. Therapeutic potential of young green barley leaves in prevention and treatment of chronic diseases: an overview. Am J Chin Med. 2015; 43(7):1311–1329.
Article15. Byun AR, Chun H, Lee J, Lee SW, Lee HS, Shim KW. Effects of a dietary supplement with barley sprout extract on blood cholesterol metabolism. Evid Based Complement Alternat Med. 2015; 2015:473056.
Article16. Seo WD, Yuk HJ, Curtis-Long MJ, Jang KC, Lee JH, Han SI, et al. Effect of the growth stage and cultivar on policosanol profiles of barley sprouts and their adenosine 5′-monophosphate-activated protein kinase activation. J Agric Food Chem. 2013; 61(5):1117–1123.
Article17. Meng TX, Irino N, Kondo R. Melanin biosynthesis inhibitory activity of a compound isolated from young green barley (Hordeum vulgare L.) in B16 melanoma cells. J Nat Med. 2015; 69(3):427–431.
Article18. Kamiyama M, Shibamoto T. Flavonoids with potent antioxidant activity found in young green barley leaves. J Agric Food Chem. 2012; 60(25):6260–6267.
Article19. Hosoi J, Abe E, Suda T, Kuroki T. Regulation of melanin synthesis of B16 mouse melanoma cells by 1 alpha, 25-dihydroxyvitamin D3 and retinoic acid. Cancer Res. 1985; 45(4):1474–1478.20. Lee KT, Kim BJ, Kim JH, Heo MY, Kim HP. Biological screening of 100 plant extracts for cosmetic use (I): inhibitory activities of tyrosinase and DOPA auto-oxidation. Int J Cosmet Sci. 1997; 19(6):291–298.
Article21. Boo YC. p-Coumaric acid as a skin whitening agent: novel findings on its ability to attenuate melanin synthesis. H&PC Today. 2013; 8(1):34–36.22. Jun HJ, Lee JH, Cho BR, Seo WD, Kim DW, Cho KJ, et al. p-Coumaric acid inhibition of CREB phosphorylation reduces cellular melanogenesis. Eur Food Res Technol. 2012; 235(6):1207–1211.
Article23. Lim JY, Ishiguro K, Kubo I. Tyrosinase inhibitory p-coumaric acid from ginseng leaves. Phytother Res. 1999; 13(5):371–375.
Article24. Park HJ, Cho JH, Hong SH, Kim DH, Jung HY, Kang IK, et al. Whitening and anti-wrinkle activities of ferulic acid isolated from Tetragonia tetragonioides in B16F10 melanoma and CCD-986sk fibroblast cells. J Nat Med. 2018; 72(1):127–135.
Article25. Maruyama H, Kawakami F, Lwin TT, Imai M, Shamsa F. Biochemical characterization of ferulic acid and caffeic acid which effectively inhibit melanin synthesis via different mechanisms in B16 melanoma cells. Biol Pharm Bull. 2018; 41(5):806–810.26. Ha DH, Choi YJ, Yoo SM. Effects of vanillic acid on the cell viability and melanogenesis in cultured human skin melanoma cells damaged by ROS-induced cytotoxicity. J Exp Biomed Sci. 2007; 13(4):349–354.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antioxidative Activity and Anti-melanogenic Effect of the Extract from the Leaves of Robinia Pseudo-acacia L
- Antaroide, a Novel Natural Nine-Membered Macrolide, Inhibits Melanin Biosynthesis in B16F10 Murine Melanoma Cells
- Comparison of whitening effect of Rubus coreanus fruit according to maturity
- The Growth Inhibitory Effect on B16F10 Melanoma Cells by 4-BPCA, an Amide Derivative of Caffeic Acid
- Anti-Melanogenic Effect from Submerged Mycelial Cultures of Ganoderma weberianum