Brain Tumor Res Treat.
2019 Apr;7(1):39-43. 10.14791/btrt.2019.7.e24.
Extensive Pachymeningeal Dissemination of Glioblastoma Mimicking Chronic Subdural Hematoma: A Case Report
- Affiliations
-
- 1Division of Neurooncology and Department of Neurosurgery, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea. yzkim@skku.edu
- 2Department of Pathology, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea.
- KMID: 2444794
- DOI: http://doi.org/10.14791/btrt.2019.7.e24
Abstract
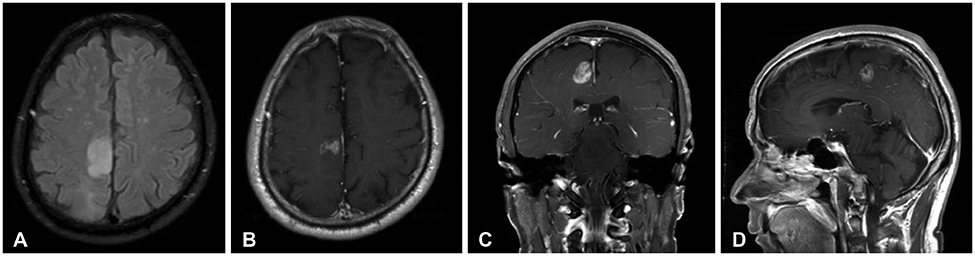
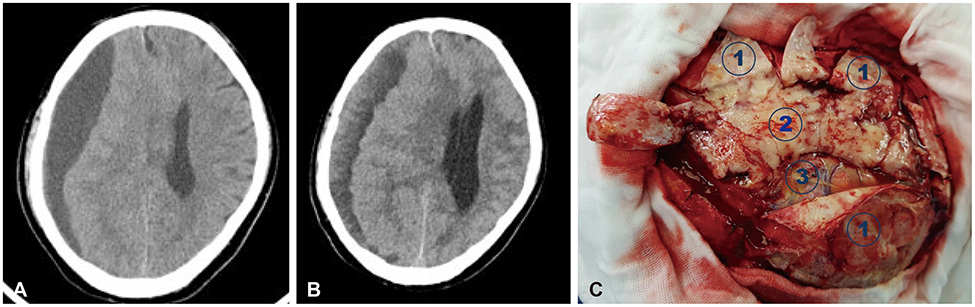
- Meningeal dissemination (MDS) of glioblastoma is rare, although its incidence might have been underestimated. MDS of glioblastoma has a fatal course. Thus, rapid and precise diagnosis of MDS is important for further palliative treatment. Unfortunately, MDS of glioblastoma could be diagnosed at a delayed time, causing failure to treat patient optimally. Herein, we present a case of a 56-year-old male with MDS of glioblastoma mimicking chronic subdural hemorrhage (CSDH) after head trauma due to slip down. During treatment for CSDH, MDS of glioblastoma was not controlled appropriately. The patient succumbed to MDS of glioblastoma at 9 weeks after the date of diagnosis of CSDH which could be an MDS.
Keyword
MeSH Terms
Figure
Reference
-
1. Dho YS, Jung KW, Ha J, et al. An updated nationwide epidemiology of primary brain tumors in Republic of Korea, 2013. Brain Tumor Res Treat. 2017; 5:16–23.
Article2. Louis DN, Perry A, Burger P, et al. International Society of Neuropathology--Haarlem consensus guidelines for nervous system tumor classification and grading. Brain Pathol. 2014; 24:429–435.
Article3. Ostrom QT, Gittleman H, Truitt G, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol. 2018; 20:suppl_4. iv1–iv86.
Article4. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016; 131:803–820.
Article5. Preusser M, de Ribaupierre S, Wöhrer A, et al. Current concepts and management of glioblastoma. Ann Neurol. 2011; 70:9–21.
Article6. Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009; 10:459–466.7. Kumar R, Jain R, Tandon V. Thalamic glioblastoma with cerebrospinal fluid dissemination in the peritoneal cavity. Pediatr Neurosurg. 1999; 31:242–245.
Article8. Salazar OM, Rubin P. The spread of glioblastoma multiforme as a determining factor in the radiation treated volume. Int J Radiat Oncol Biol Phys. 1976; 1:627–637.
Article9. Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990; 8:1277–1280.
Article10. Bryan P. CSF seeding of intra-cranial tumours: a study of 96 cases. Clin Radiol. 1974; 25:355–360.
Article11. Saito R, Kumabe T, Jokura H, Shirane R, Yoshimoto T. Symptomatic spinal dissemination of malignant astrocytoma. J Neurooncol. 2003; 61:227–235.12. Vertosick FT Jr, Selker RG. Brain stem and spinal metastases of supratentorial glioblastoma multiforme: a clinical series. Neurosurgery. 1990; 27:516–521.
Article13. Lim DA, Cha S, Mayo MC, et al. Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro Oncol. 2007; 9:424–429.
Article14. Lawton CD, Nagasawa DT, Yang I, Fessler RG, Smith ZA. Leptomeningeal spinal metastases from glioblastoma multiforme: treatment and management of an uncommon manifestation of disease. J Neurosurg Spine. 2012; 17:438–448.
Article15. Kato H, Fujimura M, Kumabe T, Ishioka C, Kanamaru R, Yoshimoto T. PTEN gene mutation and high MIB-1 labeling index may contribute to dissemination in patients with glioblastoma. J Clin Neurosci. 2004; 11:37–41.
Article16. Korshunov A, Sycheva R, Golanov A, Pronin I. Gains at the 1p36 chromosomal region are associated with symptomatic leptomeningeal dissemination of supratentorial glioblastomas. Am J Clin Pathol. 2007; 127:585–590.
Article17. Sato A, Sakurada K, Kumabe T, et al. Association of stem cell marker CD133 expression with dissemination of glioblastomas. Neurosurg Rev. 2010; 33:175–183.
Article18. Arita N, Taneda M, Hayakawa T. Leptomeningeal dissemination of malignant gliomas. Incidence, diagnosis and outcome. Acta Neurochir (Wien). 1994; 126:84–92.
Article19. Onda K, Tanaka R, Takahashi H, Takeda N, Ikuta F. Cerebral glioblastoma with cerebrospinal fluid dissemination: a clinicopathological study of 14 cases examined by complete autopsy. Neurosurgery. 1989; 25:533–540.
Article20. Lomax AJ, Yannakou CK, Rosenthal MA. Spinal cord metastasis in a patient treated with bevacizumab for glioblastoma. Target Oncol. 2013; 8:153–155.
Article21. Bae JS, Yang SH, Yoon WS, Kang SG, Hong YK, Jeun SS. The clinical features of spinal leptomeningeal dissemination from malignant gliomas. J Korean Neurosurg Soc. 2011; 49:334–338.
Article22. Grah JJ, Katalinic D, Stern-Padovan R, et al. Leptomeningeal and intramedullary metastases of glioblastoma multiforme in a patient reoperated during adjuvant radiochemotherapy. World J Surg Oncol. 2013; 11:55.
Article23. Taschner CA, Brendecke S, Weyerbrock A, Egger K, Prinz M. Freiburg neuropathology case conference: widespread mass lesions after resection of a glioblastoma multiforme. Clin Neuroradiol. 2012; 22:375–380.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chronic Subdural Hematoma Superimposed on Posttraumatic Subdural Hygroma: A Report of Three Cases
- Bilateral Acute Subdural Hematoma Following Evacuation of Chronic Subdural Hematoma
- Treatment of Chronic Subdural Hematoma with Arachnoid Cyst
- Chronic Subdural Hematoma with Calcification: Case Report
- Intraoperative Development of Contralateral Subdural Hematoma during Evacuation of Acute Subdural Hematoma: Case Report