Brain Tumor Res Treat.
2019 Apr;7(1):25-32. 10.14791/btrt.2019.7.e21.
Bacoside A Induced Sub-G0 Arrest and Early Apoptosis in Human Glioblastoma Cell Line U-87 MG through Notch Signaling Pathway
- Affiliations
-
- 1Department of Biotechnology, Dayananda Sagar College of Engineering, Bangalore, India. nraja7@gmail.com
- KMID: 2444792
- DOI: http://doi.org/10.14791/btrt.2019.7.e21
Abstract
- BACKGROUND
Glioblastoma multiforme (GBM) is a highly malignant brain tumor with a worst prognosis of less than one year despite advance treatment facilities. Among various signaling pathway genes displaying genetic modifications, aberrant expression of Notch pathway genes is frequent in GBM offering novel therapeutic targets. Herbal extracts having anticancer properties are used in adjuvant therapy that is safe and affordable as compared to chemotherapeutics. Bacopa monnieri has been used for the development of brain cells because of its neuroprotective properties. Its anticancer properties have shown to be promising in cancer treatment.
METHODS
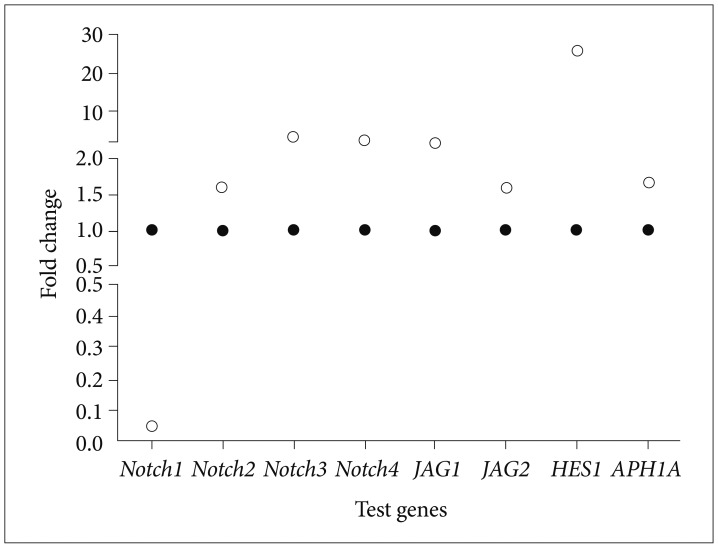
The anticancer properties of Bacoside A, an active and abundant component of Bacopa monnieri was assessed on U-87 MG cell line and its effects on expression of Notch pathway genes were studied. Cell cycle arrest and apoptosis were studied using flow cytometry. Expression of Notch pathway genes comprising of Notch receptors (notch1, notch2, notch3 and notch4), ligands (jagged1 and jagged2), a component of gamma-secretase complex (APH1A) and downstream target (HES1) were evaluated by quantitative real-time PCR.
RESULTS
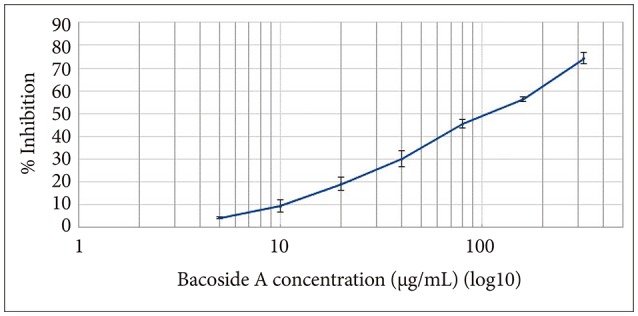
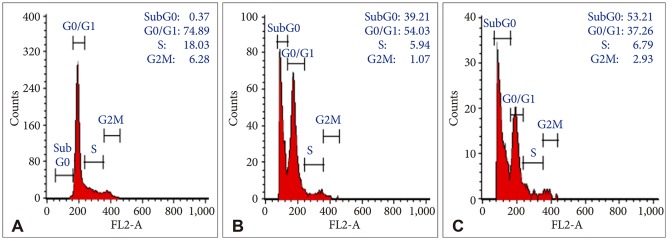
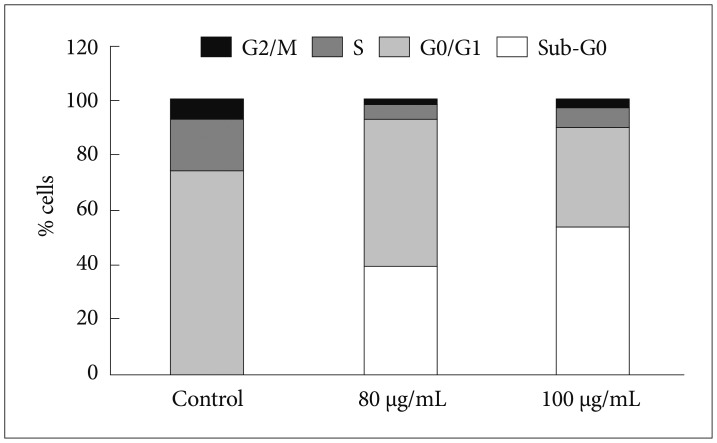
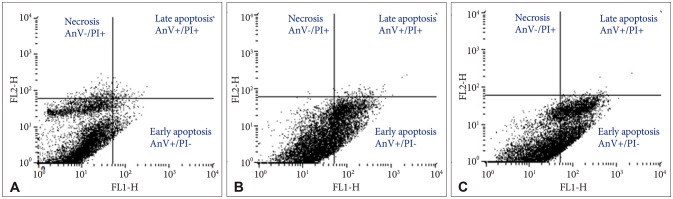
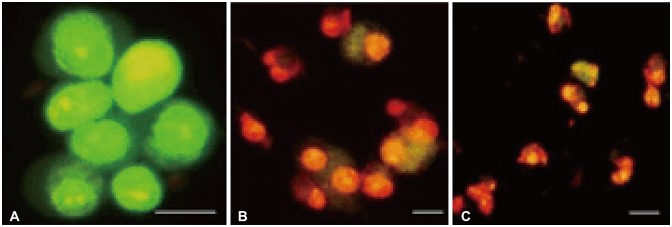
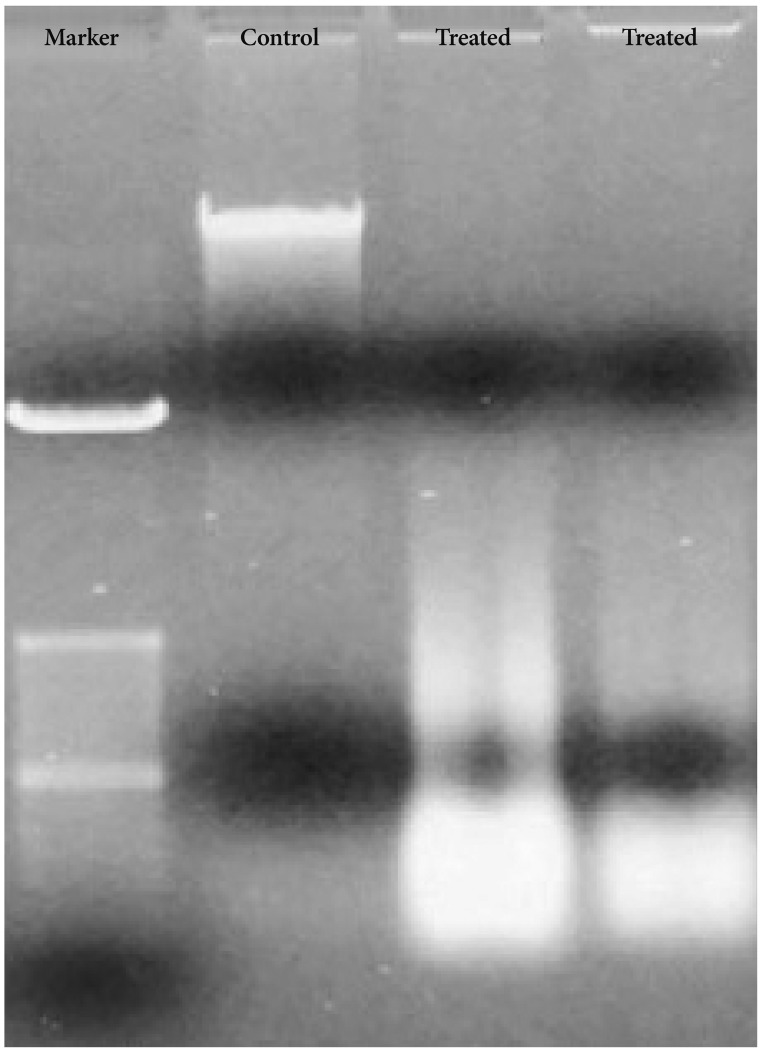
Bacoside A exhibited considerable cytotoxicity on U-87 MG cells inducing cell cycle arrest and apoptosis. Cell cycle analysis revealed a significant arrest of 39.21% cells in sub-G0 phase at 80 µg/mL concentration, increasing to 53.21% at a higher concentration of 100 µg/mL. The fraction of early apoptotic cells in control was low (3.48%) that increased substantially to 31.36% and 41.11% after 80 µg/mL and 100 µg/mL of Bacoside A treatment respectively. Additionally, the expression of notch1 gene decreased after exposure to Bacoside A with a fold change of 0.05, whereas HES1 gene expression was increased by 25 fold.
CONCLUSION
These data indicate that Bacoside A has a possible anticancer activity that could be inducing cell cycle arrest and apoptosis through Notch pathway in GBM in vitro.
Keyword
MeSH Terms
-
Amyloid Precursor Protein Secretases
Apoptosis*
Bacopa
Brain
Brain Neoplasms
Cell Cycle
Cell Cycle Checkpoints
Cell Line*
Flow Cytometry
Gene Expression
Glioblastoma*
Humans*
In Vitro Techniques
Ligands
Prognosis
Real-Time Polymerase Chain Reaction
Receptors, Notch
Amyloid Precursor Protein Secretases
Ligands
Receptors, Notch
Figure
Reference
-
1. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol. 2014; 16(Suppl 4):iv1–iv63. PMID: 25304271.
Article2. Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008; 359:492–507. PMID: 18669428.
Article3. Imayoshi I, Sakamoto M, Yamaguchi M, Mori K, Kageyama R. Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J Neurosci. 2010; 30:3489–3498. PMID: 20203209.
Article4. Axelson H. Notch signaling and cancer: emerging complexity. Semin Cancer Biol. 2004; 14:317–319. PMID: 15288256.
Article5. Hansson EM, Lendahl U, Chapman G. Notch signaling in development and disease. Semin Cancer Biol. 2004; 14:320–328. PMID: 15288257.
Article6. Dell'albani P, Rodolico M, Pellitteri R, et al. Differential patterns of NOTCH1-4 receptor expression are markers of glioma cell differentiation. Neuro Oncol. 2014; 16:204–216. PMID: 24305720.7. Narayanappa R, Rout P, Aithal MG, Chand AK. Aberrant expression of Notch1, HES1, and DTX1 genes in glioblastoma formalin-fixed paraffin-embedded tissues. Tumour Biol. 2016; 37:6935–6942. PMID: 26662803.
Article8. Purow BW, Haque RM, Noel MW, et al. Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferation. Cancer Res. 2005; 65:2353–2363. PMID: 15781650.
Article9. Chen J, Kesari S, Rooney C, et al. Inhibition of notch signaling blocks growth of glioblastoma cell lines and tumor neurospheres. Genes Cancer. 2010; 1:822–835. PMID: 21127729.
Article10. Hovinga KE, Shimizu F, Wang R, et al. Inhibition of notch signaling in glioblastoma targets cancer stem cells via an endothelial cell intermediate. Stem Cells. 2010; 28:1019–1029. PMID: 20506127.
Article11. Ridgway J, Zhang G, Wu Y, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006; 444:1083–1087. PMID: 17183323.
Article12. Anbarasi K, Vani G, Balakrishna K, Devi CS. Effect of bacoside A on brain antioxidant status in cigarette smoke exposed rats. Life Sci. 2006; 78:1378–1384. PMID: 16226278.
Article13. Simpson T, Pase M, Stough C. Bacopa monnieri as an antioxidant therapy to reduce oxidative stress in the aging brain. Evid Based Complement Alternat Med. 2015; 2015:615384. PMID: 26413126.14. Jyoti A, Sharma D. Neuroprotective role of Bacopa monniera extract against aluminium-induced oxidative stress in the hippocampus of rat brain. Neurotoxicology. 2006; 27:451–457. PMID: 16500707.
Article15. Kumar EP, Elshurafa AA, Elango K, Subburaju T, Suresh B. Cytotoxic and anti -tumour properties of ethanolic extract of bacopa monnieri (L) penn. Anc Sci Life. 1998; 17:228–234. PMID: 22556847.16. Peng L, Zhou Y, Kong de Y, Zhang WD. Antitumor activities of dammarane triterpene saponins from Bacopa monniera. Phytother Res. 2010; 24:864–868. PMID: 19960417.
Article17. Elangovan V, Govindasamy S, Ramamoorthy N, Balasubramanian K. In vitro studies on the anticancer activity of Bacopa monnieri. Fitoterapia. 1995; 66:211–215.18. Kalyani MI, Lingaraju SM, Salimath BP. A pro-apoptotic 15-kDa protein from Bacopa monnieri activates caspase-3 and downregulates Bcl-2 gene expression in mouse mammary carcinoma cells. J Nat Med. 2013; 67:123–136. PMID: 22467255.
Article19. Janani P, Sivakumari K, Geetha A, Ravisankar B, Parthasarathy C. Chemopreventive effect of bacoside A on N-nitrosodiethylamine-induced hepatocarcinogenesis in rats. J Cancer Res Clin Oncol. 2010; 136:759–770. PMID: 19916024.
Article20. Janani P, Sivakumari K, Geetha A, Yuvaraj S, Parthasarathy C. Bacoside A downregulates matrix metalloproteinases 2 and 9 in DEN-induced hepatocellular carcinoma. Cell Biochem Funct. 2010; 28:164–169. PMID: 20084675.
Article21. Prakash NS, Sundaram R, Mitra SK. In vitro and in vivo anticancer activity of Bacoside A from whole plant of Bacopa monnieri (Linn). Am J Pharmacol Toxicol. 2011; 6:11–19.22. Deepak M, Sangli GK, Arun PC, Amit A. Quantitative determination of the major saponin mixture bacoside A in Bacopa monnieri by HPLC. Phytochem Anal. 2005; 16:24–29. PMID: 15688952.23. John S, Sivakumar KC, Mishra R. Bacoside A induces tumor cell death in human glioblastoma cell lines through catastrophic macropinocytosis. Front Mol Neurosci. 2017; 10:171. PMID: 28663722.
Article24. Rauf K, Subhan F, Al-Othman AM, Khan I, Zarrelli A, Shah MR. Preclinical profile of bacopasides from Bacopa monnieri (BM) as an emerging class of therapeutics for management of chronic pains. Curr Med Chem. 2013; 20:1028–1037. PMID: 23210787.
Article25. Singh HK. Neuropsychopharmacological effects of the ayurvedic nootropic Bacopa monnieri Linn. Indian J Pharmacol. 1997; 29:S359–S365.26. Stewart ZA, Westfall MD, Pietenpol JA. Cell-cycle dysregulation and anticancer therapy. Trends Pharmacol Sci. 2003; 24:139–145. PMID: 12628359.
Article27. Nunez R. DNA measurement and cell cycle analysis by flow cytometry. Curr Issues Mol Biol. 2001; 3:67–70. PMID: 11488413.28. Kaufmann SH, Earnshaw WC. Induction of apoptosis by cancer chemotherapy. Exp Cell Res. 2000; 256:42–49. PMID: 10739650.
Article29. Cotter TG. Apoptosis and cancer: the genesis of a research field. Nat Rev Cancer. 2009; 9:501–507. PMID: 19550425.
Article30. Zhang XP, Zheng G, Zou L, et al. Notch activation promotes cell proliferation and the formation of neural stem cell-like colonies in human glioma cells. Mol Cell Biochem. 2008; 307:101–108. PMID: 17849174.
Article31. Kannan S, Sutphin RM, Hall MG, et al. Notch activation inhibits AML growth and survival: a potential therapeutic approach. J Exp Med. 2013; 210:321–337. PMID: 23359069.
Article32. Tian C, Yu Y, Jia Y, Zhu L, Zhang Y. HES1 activation suppresses proliferation of leukemia cells in acute myeloid leukemia. Ann Hematol. 2015; 94:1477–1483. PMID: 26092281.
Article33. Viatour P, Ehmer U, Saddic LA, et al. Notch signaling inhibits hepatocellular carcinoma following inactivation of the RB pathway. J Exp Med. 2011; 208:1963–1976. PMID: 21875955.
Article34. Wei G, Chang Y, Zheng J, et al. Notch1 silencing inhibits proliferation and invasion in SGC 7901 gastric cancer cells. Mol Med Rep. 2014; 9:1153–1158. PMID: 24469571.35. Ye QF, Zhang YC, Peng XQ, Long Z, Ming YZ, He LY. Silencing Notch-1 induces apoptosis and increases the chemosensitivity of prostate cancer cells to docetaxel through Bcl-2 and Bax. Oncol Lett. 2012; 3:879–884. PMID: 22741011.
Article36. Fan X, Khaki L, Zhu TS, et al. NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells. 2010; 28:5–16. PMID: 19904829.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Propranolol Inhibits the Proliferation of Human Glioblastoma Cell Lines through Notch1 and Hes1 Signaling System
- Nerve Growth Factor Stimulates Glioblastoma Proliferation through Notch1 Receptor Signaling
- Cisplatin-Induced Apoptosis by Cisplatin in Human Glioblastoma Cell Line
- Induction of Hepatocellular Carcinoma Cell Cycle Arrest and Apoptosis by Dendropanax morbifera Leveille Leaf Extract via the PI3K/AKT/mTOR Pathway
- Anti-proliferative Effect of 15,16-Dihydrotanshinone I Through Cell Cycle Arrest and the Regulation of AMP-activated Protein Kinase/Akt/mTOR and Mitogen-activated Protein Kinase Signaling Pathway in Human Hepatocellular Carcinoma Cells