World J Mens Health.
2019 May;37(2):113-127. 10.5534/wjmh.180055.
Metabolic Syndrome and Male Fertility
- Affiliations
-
- 1American Center for Reproductive Medicine, Department of Urology, Cleveland Clinic, Cleveland, OH, USA. agarwaa@ccf.org
- 2Department of Microscopy, Laboratory of Cell Biology and Unit for Multidisciplinary Research in Biomedicine, Abel Salazar Institute of Biomedical Sciences (ICBAS), University of Porto, Porto, Portugal.
- 3Department of Urology, Hamad Medical Corporation and Weill Cornell Medicine-Qatar, Doha, Qatar.
- KMID: 2443227
- DOI: http://doi.org/10.5534/wjmh.180055
Abstract
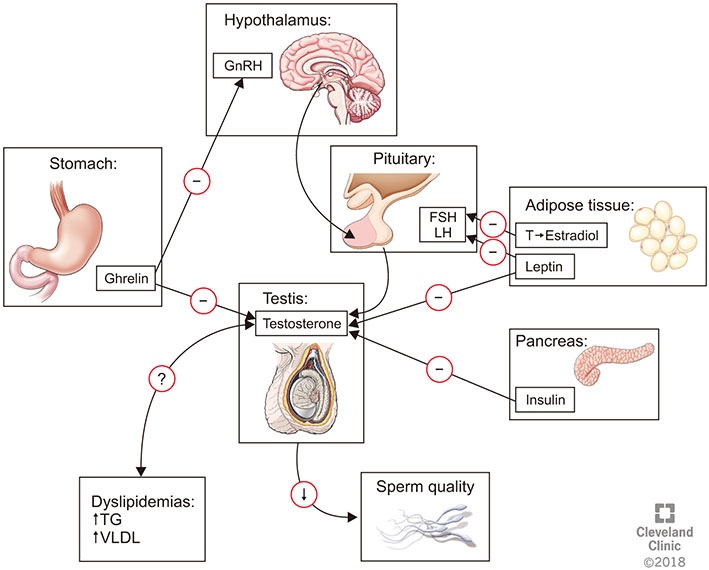
- Metabolic syndrome (MetS) represents a cluster of conditions that have a negative impact on human health overall. Its prevalence has been rapidly increasing worldwide and has coincided with a global decrease in birth rates and fertility potential. This review aims to address this observation through studying the relationship between MetS and male reproductive health. The effects of obesity, dyslipidemia, hypertension, and insulin resistance on male fertility were examined and supporting evidence explaining the pathophysiology of sperm dysfunction with each MetS component were described. Adopting a healthy lifestyle appears to be the single most important intervention to prevent the unwanted effects of MetS on men's health and fertility. Further studies addressing the components of MetS and their impact on male reproduction are required to enhance our understanding of the underlying pathophysiology and to propose new methods for therapeutic intervention.
Keyword
MeSH Terms
Figure
Reference
-
1. Kylin E. Studien ueber das Hypertonie-Hyperglyka “mie-Hyperurika” miesyndrom. Zentbl Inn Med. 1923; 44:105–127.2. Vague J. Sexual differentiation, a factor affecting the forms of obesity. Presse Med. 1947; 30:339–340.3. Haller H, Hanefeld M. Synoptische Betrachtung metabolischer Risikofaktoren. In : Haller H, Hanefeld M, Jaross W, editors. Lipidstoffwechselstörungen. Jena: Gustav Fischer Verlag;1975. p. 254–264.4. Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988; 37:1595–1607.
Article5. Kaplan NM. The deadly quartet. Upper-body obesity, glucose intolerance, hypertriglyceridemia, and hypertension. Arch Intern Med. 1989; 149:1514–1520.
Article6. Haffner SM, Valdez RA, Hazuda HP, Mitchell BD, Morales PA, Stern MP. Prospective analysis of the insulin-resistance syndrome (syndrome X). Diabetes. 1992; 41:715–722.
Article7. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome: a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006; 23:469–480.8. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of The Third Report of The National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001; 285:2486–2497.9. Consultation W. Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO consultation. Part 1. Geneva: World Health Organization;1999.10. Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR). Diabet Med. 1999; 16:442–443.11. Garber AJ, Moghissi ES, Bransome ED Jr, Clark NG, Clement S, Cobin RH, et al. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004; 10:Suppl 2. 4–9.
Article12. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998; 15:539–553.
Article13. Ahima RS. Overview of metabolic syndrome. In : Ahima RS, editor. Metabolic syndrome: a comprehensive textbook. Cham: Springer International Publishing;2016. p. 3–12.14. Falkner B, Cossrow ND. Prevalence of metabolic syndrome and obesity-associated hypertension in the racial ethnic minorities of the United States. Curr Hypertens Rep. 2014; 16:449.
Article15. Cameron AJ, Shaw JE, Zimmet PZ. The metabolic syndrome: prevalence in worldwide populations. Endocrinol Metab Clin North Am. 2004; 33:351–357.
Article16. Lotti F, Corona G, Degli Innocenti S, Filimberti E, Scognamiglio V, Vignozzi L, et al. Seminal, ultrasound and psychobiological parameters correlate with metabolic syndrome in male members of infertile couples. Andrology. 2013; 1:229–239.
Article17. Lotti F, Corona G, Vignozzi L, Rossi M, Maseroli E, Cipriani S, et al. Metabolic syndrome and prostate abnormalities in male subjects of infertile couples. Asian J Androl. 2014; 16:295–304.
Article18. Leisegang K, Udodong A, Bouic PJ, Henkel RR. Effect of the metabolic syndrome on male reproductive function: a case-controlled pilot study. Andrologia. 2014; 46:167–176.
Article19. Ventimiglia E, Capogrosso P, Colicchia M, Boeri L, Serino A, Castagna G, et al. Metabolic syndrome in white European men presenting for primary couple's infertility: investigation of the clinical and reproductive burden. Andrology. 2016; 4:944–951.
Article20. Ehala-Aleksejev K, Punab M. The effect of metabolic syndrome on male reproductive health: a cross-sectional study in a group of fertile men and male partners of infertile couples. PLoS One. 2018; 13:e0194395.
Article21. Pozza C, Isidori AM. What's behind the obesity epidemic. Cham: Imaging in Bariatric Surgery, Springer International Publishing AG;2018. p. 1–8.22. World Health Organization. Obesity and overweight fact sheet [Internet]. Geneva: World Health Organization;c2016. cited 2018 Sep. Available from: http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.23. Gadde KM, Martin CK, Berthoud HR, Heymsfield SB. Obesity: pathophysiology and management. J Am Coll Cardiol. 2018; 71:69–84.24. Okosun IS, Liao Y, Rotimi CN, Prewitt TE, Cooper RS. Abdominal adiposity and clustering of multiple metabolic syndrome in White, Black and Hispanic Americans. Ann Epidemiol. 2000; 10:263–270.
Article25. Kaila B, Raman M. Obesity: a review of pathogenesis and management strategies. Can J Gastroenterol. 2008; 22:61–68.
Article26. Prentice AM, Jebb SA. Beyond body mass index. Obes Rev. 2001; 2:141–147.
Article27. Panuganti KK, Lenehan CP. Obesity. Treasure Island (FL): StatPearls;2017.28. Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995; 269:543–546.
Article29. Villanueva EC, Myers MG Jr. Leptin receptor signaling and the regulation of mammalian physiology. Int J Obes (Lond). 2008; 32:Suppl 7. S8–S12.
Article30. Martin SS, Qasim A, Reilly MP. Leptin resistance: a possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J Am Coll Cardiol. 2008; 52:1201–1210.31. Crujeiras AB, Carreira MC, Cabia B, Andrade S, Amil M, Casanueva FF. Leptin resistance in obesity: an epigenetic landscape. Life Sci. 2015; 140:57–63.
Article32. Tschöp M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001; 50:707–709.
Article33. Valassi E, Scacchi M, Cavagnini F. Neuroendocrine control of food intake. Nutr Metab Cardiovasc Dis. 2008; 18:158–168.
Article34. Alvarez Bartolomé M, Borque M, Martinez-Sarmiento J, Aparicio E, Hernández C, Cabrerizo L, et al. Peptide YY secretion in morbidly obese patients before and after vertical banded gastroplasty. Obes Surg. 2002; 12:324–327.
Article35. Murphy KG, Bloom SR. Gut hormones and the regulation of energy homeostasis. Nature. 2006; 444:854–859.
Article36. O'Keefe JH, Gheewala NM, O'Keefe JO. Dietary strategies for improving post-prandial glucose, lipids, inflammation, and cardiovascular health. J Am Coll Cardiol. 2008; 51:249–255.37. Egger G, Dixon J. Inflammatory effects of nutritional stimuli: further support for the need for a big picture approach to tackling obesity and chronic disease. Obes Rev. 2010; 11:137–149.
Article38. Gallagher EJ, LeRoith D. Obesity and diabetes: the increased risk of cancer and cancer-related mortality. Physiol Rev. 2015; 95:727–748.
Article39. Sermondade N, Faure C, Fezeu L, Shayeb AG, Bonde JP, Jensen TK, et al. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum Reprod Update. 2013; 19:221–231.
Article40. Tang WH, Zhuang XJ, Ma LL, Qiao J, Hong K, Zhao LM, et al. Correlation between body mass index and semen quality in male infertility patients. Turk J Med Sci. 2015; 45:1300–1305.
Article41. Alshahrani S, Ahmed AF, Gabr AH, Abalhassan M, Ahmad G. The impact of body mass index on semen parameters in infertile men. Andrologia. 2016; 48:1125–1129.
Article42. Bieniek JM, Kashanian JA, Deibert CM, Grober ED, Lo KC, Brannigan RE, et al. Influence of increasing body mass index on semen and reproductive hormonal parameters in a multi-institutional cohort of subfertile men. Fertil Steril. 2016; 106:1070–1075.
Article43. Dupont C, Faure C, Sermondade N, Boubaya M, Eustache F, Clément P, et al. Obesity leads to higher risk of sperm DNA damage in infertile patients. Asian J Androl. 2013; 15:622–625.
Article44. Fariello RM, Pariz JR, Spaine DM, Cedenho AP, Bertolla RP, Fraietta R. Association between obesity and alteration of sperm DNA integrity and mitochondrial activity. BJU Int. 2012; 110:863–867.
Article45. M Al-Ali B, Gutschi T, Pummer K, Zigeuner R, Brookman-May S, Wieland WF, et al. Body mass index has no impact on sperm quality but on reproductive hormones levels. Andrologia. 2014; 46:106–111.
Article46. Macdonald AA, Stewart AW, Farquhar CM. Body mass index in relation to semen quality and reproductive hormones in New Zealand men: a cross-sectional study in fertility clinics. Hum Reprod. 2013; 28:3178–3187.
Article47. Andersen JM, Herning H, Aschim EL, Hjelmesæth J, Mala T, Hanevik HI, et al. Body mass index is associated with impaired semen characteristics and reduced levels of anti-müllerian hormone across a wide weight range. PLoS One. 2015; 10:e0130210.
Article48. Repaci A, Pasquali R. Reproductive disorders and obesity in males and females and focus on the polycystic ovary syndrome. Metabolic syndrome: a comprehensive textbook. Cham: Springer International Publishing AG;2014. p. 1–19.49. O'Shaughnessy PJ. Hormonal control of germ cell development and spermatogenesis. Semin Cell Dev Biol. 2014; 29:55–65.50. Vermeulen A, Kaufman JM, Deslypere JP, Thomas G. Attenuated luteinizing hormone (LH) pulse amplitude but normal LH pulse frequency, and its relation to plasma androgens in hypogonadism of obese men. J Clin Endocrinol Metab. 1993; 76:1140–1146.
Article51. George JT, Millar RP, Anderson RA. Hypothesis: kisspeptin mediates male hypogonadism in obesity and type 2 diabetes. Neuroendocrinology. 2010; 91:302–307.
Article52. Shafik A, Olfat S. Scrotal lipomatosis. Br J Urol. 1981; 53:50–54.53. Shafik A, Olfat S. Lipectomy in the treatment of scrotal lipomatosis. Br J Urol. 1981; 53:55–61.
Article54. Kodama H, Yamaguchi R, Fukuda J, Kasai H, Tanaka T. Increased oxidative deoxyribonucleic acid damage in the spermatozoa of infertile male patients. Fertil Steril. 1997; 68:519–524.
Article55. Sengenès C, Miranville A, Lolmède K, Curat CA, Bouloumié A. The role of endothelial cells in inflamed adipose tissue. J Intern Med. 2007; 262:415–421.
Article56. Rzheshevsky AV. Fatal “triad”: lipotoxicity, oxidative stress, and phenoptosis. Biochemistry (Mosc). 2013; 78:991–1000.
Article57. Feldman HA, Johannes CB, Derby CA, Kleinman KP, Mohr BA, Araujo AB, et al. Erectile dysfunction and coronary risk factors: prospective results from the Massachusetts male aging study. Prev Med. 2000; 30:328–338.
Article58. Burnett AL, Strong TD, Trock BJ, Jin L, Bivalacqua TJ, Musicki B. Serum biomarker measurements of endothelial function and oxidative stress after daily dosing of sildenafil in type 2 diabetic men with erectile dysfunction. J Urol. 2009; 181:245–251.
Article59. Araña Rosaínz Mde J, Ojeda MO, Acosta JR, Elías-Calles LC, González NO, Herrera OT, et al. Imbalanced low-grade inflammation and endothelial activation in patients with type 2 diabetes mellitus and erectile dysfunction. J Sex Med. 2011; 8:2017–2030.60. Mergenthaler P, Lindauer U, Dienel GA, Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 2013; 36:587–597.
Article61. Rato L, Alves MG, Socorro S, Duarte AI, Cavaco JE, Oliveira PF. Metabolic regulation is important for spermatogenesis. Nat Rev Urol. 2012; 9:330–338.
Article62. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care. 2018; 41:S13–S27.63. Skyler JS, Bakris GL, Bonifacio E, Darsow T, Eckel RH, Groop L, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes. 2017; 66:241–255.
Article64. Lutz W. Fertility rates and future population trends: will Europe's birth rate recover or continue to decline? Int J Androl. 2006; 29:25–33.
Article65. Rama Raju GA, Jaya Prakash G, Murali Krishna K, Madan K, Siva Narayana T, Ravi Krishna CH. Noninsulin-dependent diabetes mellitus: effects on sperm morphological and functional characteristics, nuclear DNA integrity and outcome of assisted reproductive technique. Andrologia. 2012; 44:Suppl 1. 490–498.
Article66. An T, Wang YF, Liu JX, Pan YY, Liu YF, He ZC, et al. Comparative analysis of proteomes between diabetic and normal human sperm: insights into the effects of diabetes on male reproduction based on the regulation of mitochondria-related proteins. Mol Reprod Dev. 2018; 85:7–16.
Article67. Singh AK, Tomarz S, Chaudhari AR, Sinqh R, Verma N. Type 2 diabetes mellitus affects male fertility potential. Indian J Physiol Pharmacol. 2014; 58:403–406.68. Bhattacharya SM, Ghosh M, Nandi N. Diabetes mellitus and abnormalities in semen analysis. J Obstet Gynaecol Res. 2014; 40:167–171.
Article69. Jangir RN, Jain GC. Diabetes mellitus induced impairment of male reproductive functions: a review. Curr Diabetes Rev. 2014; 10:147–157.
Article70. Oliveira PF, Alves MG, Rato L, Silva J, Sá R, Barros A, et al. Influence of 5α-dihydrotestosterone and 17β-estradiol on human Sertoli cells metabolism. Int J Androl. 2011; 34:e612–e620.
Article71. Alves MG, Rato L, Carvalho RA, Moreira PI, Socorro S, Oliveira PF. Hormonal control of Sertoli cell metabolism regulates spermatogenesis. Cell Mol Life Sci. 2013; 70:777–793.
Article72. Seethalakshmi L, Menon M, Diamond D. The effect of streptozotocin-induced diabetes on the neuroendocrine-male reproductive tract axis of the adult rat. J Urol. 1987; 138:190–194.
Article73. Shrilatha B, Muralidhara . Early oxidative stress in testis and epididymal sperm in streptozotocin-induced diabetic mice: its progression and genotoxic consequences. Reprod Toxicol. 2007; 23:578–587.
Article74. Beatrice AM, Dutta D, Kumar M, Kumbenahalli Siddegowda S, Sinha A, Ray S, et al. Testosterone levels and type 2 diabetes in men: current knowledge and clinical implications. Diabetes Metab Syndr Obes. 2014; 7:481–486.75. Walker WH. Molecular mechanisms of testosterone action in spermatogenesis. Steroids. 2009; 74:602–607.
Article76. Burke JP, Jacobson DJ, McGree ME, Nehra A, Roberts RO, Girman CJ, et al. Diabetes and sexual dysfunction: results from the Olmsted County study of urinary symptoms and health status among men. J Urol. 2007; 177:1438–1442.
Article77. Fedder J, Kaspersen MD, Brandslund I, Højgaard A. Retro-grade ejaculation and sexual dysfunction in men with diabetes mellitus: a prospective, controlled study. Andrology. 2013; 1:602–606.78. Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003; 26:1553–1579.
Article79. De Young L, Yu D, Bateman RM, Brock GB. Oxidative stress and antioxidant therapy: their impact in diabetes-associated erectile dysfunction. J Androl. 2004; 25:830–836.
Article80. Herman A, Adar R, Rubinstein Z. Vascular lesions associated with impotence in diabetic and nondiabetic arterial occlusive disease. Diabetes. 1978; 27:975–981.
Article81. Blanco R, Saenz de Tejada I, Goldstein I, Krane RJ, Wotiz HH, Cohen RA. Dysfunctional penile cholinergic nerves in diabetic impotent men. J Urol. 1990; 144:278–280.
Article82. Musicki B, Burnett AL. Endothelial dysfunction in diabetic erectile dysfunction. Int J Impot Res. 2007; 19:129–138.
Article83. Saenz de Tejada I, Goldstein I, Azadzoi K, Krane RJ, Cohen RA. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. N Engl J Med. 1989; 320:1025–1030.
Article84. Catapano AL, Reiner Z, De Backer G, Graham I, Taskinen MR, Wiklund O, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Atherosclerosis. 2011; 217:Suppl 1. S1–S44.85. Longuet C, Sinclair EM, Maida A, Baggio LL, Maziarz M, Charron MJ, et al. The glucagon receptor is required for the adaptive metabolic response to fasting. Cell Metab. 2008; 8:359–371.
Article86. Pégorier JP, Le May C, Girard J. Control of gene expression by fatty acids. J Nutr. 2004; 134:2444S–2449S.87. Schisterman EF, Mumford SL, Browne RW, Barr DB, Chen Z, Louis GM. Lipid concentrations and couple fecundity: the LIFE study. J Clin Endocrinol Metab. 2014; 99:2786–2794.
Article88. Hagiuda J, Ishikawa H, Furuuchi T, Hanawa Y, Marumo K. Relationship between dyslipidaemia and semen quality and serum sex hormone levels: an infertility study of 167 Japanese patients. Andrologia. 2014; 46:131–135.
Article89. Ergün A, Köse SK, Aydos K, Ata A, Avci A. Correlation of seminal parameters with serum lipid profile and sex hormones. Arch Androl. 2007; 53:21–23.
Article90. Shalaby MA, el-Zorba HY, Kamel GM. Effect of alpha-to-copherol and simvastatin on male fertility in hypercholesterolemic rats. Pharmacol Res. 2004; 50:137–142.
Article91. O'Shea PM, Griffin TP, Fitzgibbon M. Hypertension: the role of biochemistry in the diagnosis and management. Clin Chim Acta. 2017; 465:131–143.92. Guo D, Li S, Behr B, Eisenberg ML. Hypertension and male fertility. World J Mens Health. 2017; 35:59–64.
Article93. Eisenberg ML, Li S, Behr B, Pera RR, Cullen MR. Relationship between semen production and medical comorbidity. Fertil Steril. 2015; 103:66–71.
Article94. Svartberg J, von Mühlen D, Schirmer H, Barrett-Connor E, Sundfjord J, Jorde R. Association of endogenous testosterone with blood pressure and left ventricular mass in men. The Tromsø Study. Eur J Endocrinol. 2004; 150:65–71.95. Fogari R, Zoppi A, Preti P, Rinaldi A, Marasi G, Vanasia A, et al. Sexual activity and plasma testosterone levels in hypertensive males. Am J Hypertens. 2002; 15:217–221.
Article96. Burchardt M, Burchardt T, Baer L, Kiss AJ, Pawar RV, Shabsigh A, et al. Hypertension is associated with severe erectile dysfunction. J Urol. 2000; 164:1188–1191.
Article97. Thompson IM, Tangen CM, Goodman PJ, Probstfield JL, Moinpour CM, Coltman CA. Erectile dysfunction and subsequent cardiovascular disease. JAMA. 2005; 294:2996–3002.98. Ponholzer A, Temml C, Mock K, Marszalek M, Obermayr R, Madersbacher S. Prevalence and risk factors for erectile dysfunction in 2869 men using a validated questionnaire. Eur Urol. 2005; 47:80–85. discussion 85-6.
Article99. Toblli JE, Stella I, Inserra F, Ferder L, Zeller F, Mazza ON. Morphological changes in cavernous tissue in spontaneously hypertensive rats. Am J Hypertens. 2000; 13:686–692.
Article100. Ushiyama M, Morita T, Kuramochi T, Yagi S, Katayama S. Erectile dysfunction in hypertensive rats results from impairment of the relaxation evoked by neurogenic carbon monoxide and nitric oxide. Hypertens Res. 2004; 27:253–261.
Article101. Håkonsen LB, Thulstrup AM, Aggerholm AS, Olsen J, Bonde JP, Andersen CY, et al. Does weight loss improve semen quality and reproductive hormones? Results from a cohort of severely obese men. Reprod Health. 2011; 8:24.
Article102. Jaffar M. Does weight loss improve fertility with respect to semen parameters? Results from a large cohort study. Int J Infertil Fetal Med. 2016; 7:94–99.103. Reis LO, Zani EL, Saad RD, Chaim EA, de Oliveira LC, Fregonesi A. Bariatric surgery does not interfere with sperm quality: a preliminary long-term study. Reprod Sci. 2012; 19:1057–1062.104. Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004; 292:1724–1737.
Article105. El Bardisi H, Majzoub A, Arafa M, AlMalki A, Al Said S, Khalafalla K, et al. Effect of bariatric surgery on semen parameters and sex hormone concentrations: a prospective study. Reprod Biomed Online. 2016; 33:606–611.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Metabolic Syndrome and Male Infertility
- Letter: Association between Exercise and Metabolic Syndrome in Koreans (J Obes Metab Syndr 2018;27:117-24)
- Comparison of fertility competence in toll-like receptor 4 (TLR4)-knock out male mice fed a high-fat diet
- Relationship Between Long Working Hours and Metabolic Syndrome Among Korean Workers
- Journal of Obesity & Metabolic Syndrome: A New International Journal Targeting the Pathophysiology and Treatment of Obesity and Metabolic Syndrome