Pediatr Infect Vaccine.
2019 Apr;26(1):1-10. 10.14776/piv.2019.26.e1.
Antibiotics Susceptability of Streptococcus pneumoniae Isolated from Single Tertiary Childrens' Hospital Since 2014 and Choice of Appropriate Empirical Antibiotics
- Affiliations
-
- 1Department of Pediatrics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, the Republic of Korea. entier@amc.seoul.kr
- 2Department of Laboratory Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, the Republic of Korea.
- KMID: 2443160
- DOI: http://doi.org/10.14776/piv.2019.26.e1
Abstract
- PURPOSE
We investigated the distribution and antimicrobial resistance of pneumococcal isolates from hospitalized children at Asan Medical Center for recent 4 years, and aimed to recommend proper choice of empirical antibiotics for pneumococcal infection.
METHODS
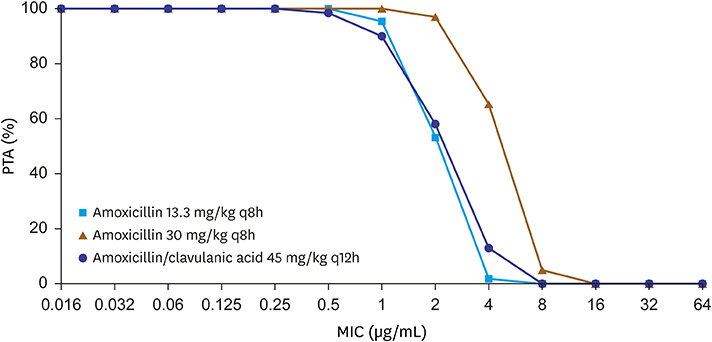
From March 2014 to May 2018, children admitted to Asan Medical Center Childrens' Hospital with pneumococcal infection were subjected for evaluation of minimal inhibitory concentration (MIC) for β-lactams and macrolide antibiotics. Patient's age, underlying disease, gender were retrospectively collected. Using Monte Carlo simulation model and MIC from our study, we predicted the rate of treatment success with amoxicillin treatment.
RESULTS
Sixty-three isolates were analyzed including 20.6% (n=13) of invasive isolates, and 79.4% (n=50) of non-invasive isolates; median age were 3.3 years old, and 87.3% of the pneumococcal infections occurred to children with underlying disease. Overall susceptibility rate was 49.2%, 68.2%, and 74.6% for amoxicillin, parenteral penicillin, and cefotaxime respectively. 23.8% and 9.5% of the isolates showed high resistance for amoxicillin, and cefotaxime. Only 4.8% (n=3) were susceptible to erythromycin. Monte Carlo simulation model revealed the likelihood of treatment success was 46.0% at the dosage of 90 mg/kg/day of amoxicillin.
CONCLUSIONS
Recent pneumococcal isolates from pediatric patients with underlying disease revealed high resistance for amoxicillin and cefotaxime, and high resistance for erythromycin. Prudent choice of antibiotics based on the local data of resistance cannot be emphasized enough, especially in high risk patients with underlying disease, and timely vaccination should be implemented for prevention of the spread of resistant strains.
Keyword
MeSH Terms
Figure
Reference
-
1. Kim KH, Sohn YM, Kang JH, Kim KN, Kim DS, Kim JH, et al. The causative organisms of bacterial meningitis in Korean children, 1986–1995. J Korean Med Sci. 1998; 13:60–64.
Article2. Hausdorff WP, Feikin DR, Klugman KP. Epidemiological differences among pneumococcal serotypes. Lancet Infect Dis. 2005; 5:83–93.
Article3. Tan TQ. Pediatric invasive pneumococcal disease in the United States in the era of pneumococcal conjugate vaccines. Clin Microbiol Rev. 2012; 25:409–419.
Article4. Brandileone MC, Vieira VS, Casagrande ST, Zanella RC, Guerra ML, Bokermann S, et al. Prevalence of serotypes and antimicrobial resistance of Streptococcus pneumoniae strains isolated from Brazilian children with invasive infections. Pneumococcal study group in Brazil for the SIREVA project. Regional system for vaccines in Latin America. Microb Drug Resist. 1997; 3:141–146.5. Mantese OC, Paula A, Almeida VV, Aguiar PA, Wolkers PC, Alvares JR, et al. Prevalence of serotypes and antimicrobial resistance of invasive strains of pneumococcus in children: analysis of 9 years. J Pediatr (Rio J). 2009; 85:495–502.
Article6. Klugman KP. Pneumococcal resistance to antibiotics. Clin Microbiol Rev. 1990; 3:171–196.
Article7. Song EK, Lee JH, Kim NH, Lee JA, Kim DH, Park KW, et al. Epidemiology and clinical features of invasive pneumococcal infections in children. Korean J Pediatr Infect Dis. 2005; 12:140–148.
Article8. Doern GV, Richter SS, Miller A, Miller N, Rice C, Heilmann K, et al. Antimicrobial resistance among Streptococcus pneumoniae in the United States: have we begun to turn the corner on resistance to certain antimicrobial classes? Clin Infect Dis. 2005; 41:139–148.
Article9. Song JH, Lee NY, Ichiyama S, Yoshida R, Hirakata Y, Fu W, et al. Spread of drug-resistant Streptococcus pneumoniae in Asian countries: Asian Network for Surveillance of Resistant Pathogens (ANSORP) Study. Clin Infect Dis. 1999; 28:1206–1211.
Article10. Weinstein MP, Klugman KP, Jones RN. Rationale for revised penicillin susceptibility breakpoints versus Streptococcus pneumoniae: coping with antimicrobial susceptibility in an era of resistance. Clin Infect Dis. 2009; 48:1596–1600.
Article11. CLSI. Performance standards for antimicrobial susceptibility testing: eighteenth informational supplement. CLSI document M100-S26. Wayne: Clinical and Laboratory Standards Institute;2008.12. Cho EY, Lee H, Choi EH, Kim YJ, Eun BW, Cho YK, et al. Serotype distribution and antibiotic resistance of Streptococcus pneumoniae isolated from invasive infections after optional use of the 7-valent conjugate vaccine in Korea, 2006–2010. Diagn Microbiol Infect Dis. 2014; 78:481–486.
Article13. Vuori-Holopainen E, Peltola H, Kallio MJ. SE-TU Study Group. Narrow-versus broad-spectrum parenteral anatimicrobials against common infections of childhood: a prospective and randomised comparison between penicillin and cefuroxime. Eur J Pediatr. 2000; 159:878–884.
Article14. Paik JY, Choi JH, Cho EY, Oh CE, Lee J, Choi EH, et al. Antibiotics susceptability of Streptococcus pneumoniae isolated from pharynx in healthy Korean children and choice of proper empirical oral antibiotics using pharmacokinetics/pharmacodynamics model. Korean J Pediatr Infect Dis. 2011; 18:109–116.
Article15. Fallon RM, Kuti JL, Doern GV, Girotto JE, Nicolau DP. Pharmacodynamic target attainment of oral β-lactams for the empiric treatment of acute otitis media in children. Paediatr Drugs. 2008; 10:329–335.
Article16. Jung YS. Antibiotics resistance of Streptococcus pneumoniae and Enterococcus sp. J Korean Soc Chemother. 1993; 11:48–55.17. Friedland IR, Klugman KP. Antibiotic-resistant pneumococcal disease in South African children. Am J Dis Child. 1992; 146:920–923.
Article18. Pallares R, Liñares J, Vadillo M, Cabellos C, Manresa F, Viladrich PF, et al. Resistance to penicillin and cephalosporin and mortality from severe pneumococcal pneumonia in Barcelona, Spain. N Engl J Med. 1995; 333:474–480.
Article19. Song JH, Jung SI, Ki HK, Shin MH, Ko KS, Son JS, et al. Clinical outcomes of pneumococcal pneumonia caused by antibiotic-resistant strains in Asian countries: a study by the Asian Network for Surveillance of Resistant Pathogens. Clin Infect Dis. 2004; 38:1570–1578.
Article20. Song JH, Jung SI, Ko KS, Kim NY, Son JS, Chang HH, et al. High prevalence of antimicrobial resistance among clinical Streptococcus pneumoniae isolates in Asia (an ANSORP study). Antimicrob Agents Chemother. 2004; 48:2101–2107.
Article21. Yum HY. Antibiotics for bacterial pneumonia in children. Korean J Pediatr. 2009; 52:283–288.
Article22. Torumkuney D, Chaiwarith R, Reechaipichitkul W, Malatham K, Chareonphaibul V, Rodrigues C, et al. Results from the survey of antibiotic resistance (SOAR) 2012–14 in Thailand, India, South Korea and Singapore. J Antimicrob Chemother. 2016; 71:Suppl 1. i3–i19.
Article23. Cho EY, Kang HM, Lee J, Kang JH, Choi EH, Lee HJ. Changes in serotype distribution and antibiotic resistance of nasopharyngeal isolates of Streptococcus pneumoniae from children in Korea, after optional use of the 7-valent conjugate vaccine. J Korean Med Sci. 2012; 27:716–722.
Article24. Hyde TB, Gay K, Stephens DS, Vugia DJ, Pass M, Johnson S, et al. Macrolide resistance among invasive Streptococcus pneumoniae isolates. JAMA. 2001; 286:1857–1862.
Article25. Song JH, Chang HH, Suh JY, Ko KS, Jung SI, Oh WS, et al. Macrolide resistance and genotypic characterization of Streptococcus pneumoniae in Asian countries: a study of the Asian Network for Surveillance of Resistant Pathogens (ANSORP). J Antimicrob Chemother. 2004; 53:457–463.
Article26. Robinson KA, Baughman W, Rothrock G, Barrett NL, Pass M, Lexau C, et al. Epidemiology of invasive Streptococcus pneumoniae infections in the United States, 1995–1998: opportunities for prevention in the conjugate vaccine era. JAMA. 2001; 285:1729–1735.
Article27. Hicks LA, Harrison LH, Flannery B, Hadler JL, Schaffner W, Craig AS, et al. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998–2004. J Infect Dis. 2007; 196:1346–1354.
Article28. Moore MR, Whitney CG. Emergence of nonvaccine serotypes following introduction of pneumococcal conjugate vaccine: cause and effect? Clin Infect Dis. 2008; 46:183–185.
Article29. Yun KW, Choi EH, Lee HJ, Kang JH, Kim KH, Kim DS, et al. Genetic structures of invasive Streptococcus pneumoniae isolates from Korean children obtained between 1995 and 2013. BMC Infect Dis. 2018; 18:268.
Article30. Kim SH, Bae IK, Park D, Lee K, Kim NY, Song SA, et al. Serotype distribution and antimicrobial resistance of Streptococcus pneumoniae isolates causing invasive and noninvasive pneumococcal diseases in Korea from 2008 to 2014. Biomed Res Int. 2016; 2016:6950482.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antibiotics Susceptability of Streptococcus pneumoniae Isolated from Pharynx in Healthy Korean Children and Choice of Proper Empirical Oral Antibiotics Using Pharmacokinetics/Pharmacodynamics Model
- Characteristics of Microbiology of Peritonsillar Abscess
- Microbial isolates and antibiotic sensitivity in patients hospitalized with odontogenic infections at a tertiary center over 10 years
- A Case of Bilateral Endogenous Endophthalmitis in a Streptococcus pneumoniae Meningitis Patient
- Community Acquired Pneumonia