Nat Prod Sci.
2019 Mar;25(1):16-22. 10.20307/nps.2019.25.1.16.
Anti-inflammatory Activity of Standardized Fraction from Inula helenium L. via Suppression of NF-κB Pathway in RAW 264.7 Cells
- Affiliations
-
- 1Natural Products Research Institute, College of Pharmacy, Seoul National University, Seoul 08826, Republic of Korea. kims@snu.ac.kr
- 2Current Address: Winship Cancer Institute, Emory University School of Medicine, Atlanta, GA 30322, USA.
- KMID: 2443098
- DOI: http://doi.org/10.20307/nps.2019.25.1.16
Abstract
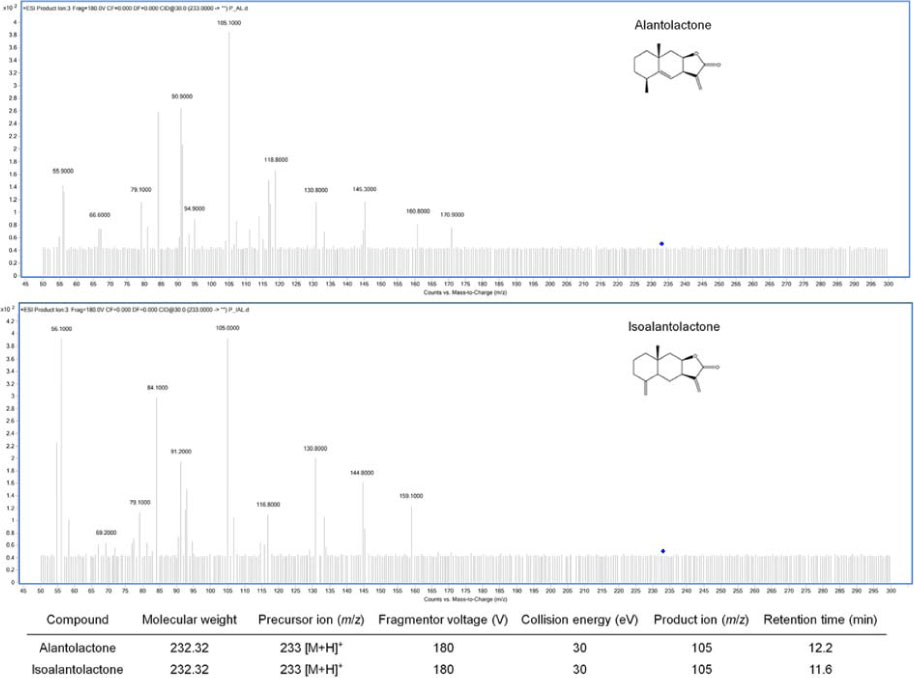
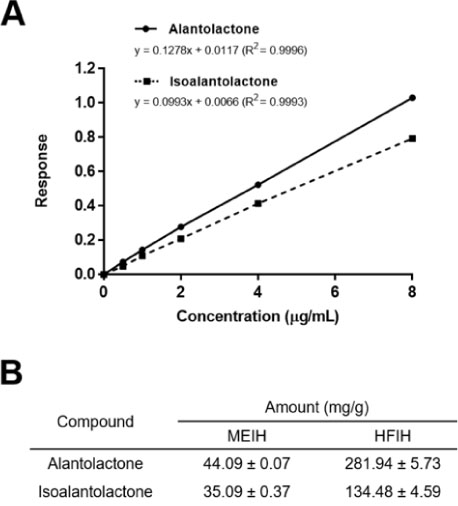
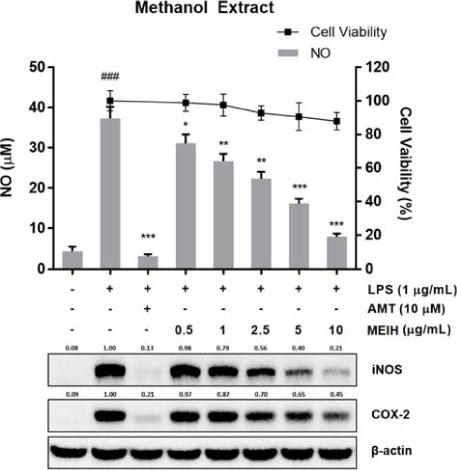
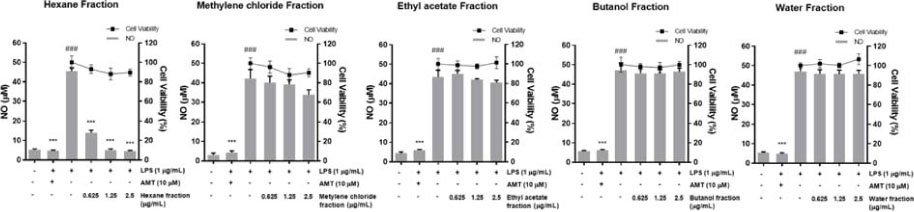
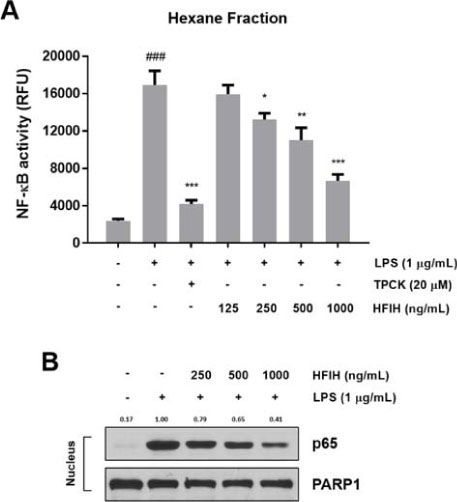
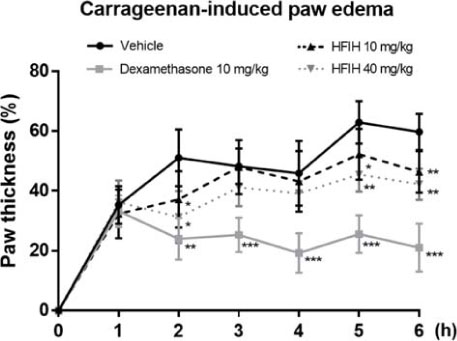
- Inula helenium L. is rich source of eudesmane-type sesquiterpene lactones, mainly alantolactone and isoalantolactone, which have the various pharmacological functions. In this study, we examined the inhibitory effects of nitric oxide (NO) production of hexane, methylene chloride, ethyl acetate, butanol, and water fractions from I. helenium and investigated the anti-inflammatory effect of hexane fraction of I. helenium (HFIH) in LPS-induced RAW 264.7 cells. Quantification of alantolactone and isoalantolactone from HFIH was carried out for the standardization by multiple reaction monitoring using triple quadrupole mass spectrometer. HFIH significantly inhibited inducible NO synthase (iNOS) and cyclooxygenase-2 (COX-2) protein as well as their downstream products NO and prostaglandin E₂ (PGE₂) in LPS-stimulated RAW 264.7 cells. Moreover, HFIH suppressed NF-κB transcriptional activity by decreasing the translocation of p65 to the nucleus. The in vivo study further confirmed that HFIH attenuated the paw edema induced by carrageenan in an acute inflammation model. These findings suggest that HFIH may be useful as a promising phytomedicine for inflammatory-associated diseases.
MeSH Terms
Figure
Reference
-
1. Fujiwara N, Kobayashi K. Curr Drug Targets Inflamm Allergy. 2005; 4:281–286.2. Amin AR, Attur M, Abramson SB. Curr Opin Rheumatol. 1999; 11:202–209.3. Tak PP, Firestein GS. J Clin Invest. 2001; 107:7–11.4. Yamamoto Y, Gaynor RB. Trends Biochem Sci. 2004; 29:72–79.5. Yuan G, Wahlqvist ML, He G, Yang M, Li D. Asia Pac J Clin Nutr. 2006; 15:143–152.6. Spiridon I, Nechita CB, Niculaua M, Silion M, Armatu A, Teaca CA, Bodirlau R. Cent Eur J Chem. 2013; 11:1699–1709.7. Trendafilova A, Chanev C, Todorova M. Pharmacogn Mag. 2010; 6:234–237.8. Babaei G, Aliarab A, Abroon S, Rasmi Y, Aziz SG. Biomed Pharmacother. 2018; 106:239–246.9. Chun J, Choi RJ, Khan S, Lee DS, Kim YC, Nam YJ, Lee DU, Kim YS. Int Immunopharmacol. 2012; 14:375–383.10. He G, Zhang X, Chen Y, Chen J, Li L, Xie Y. Biomed Pharmacother. 2017; 90:598–607.11. Ketai W, Huitao L, Yunkun Z, Xingguo C, Zhide H, Yucheng S, Xiao M. Talanta. 2000; 52:1001–1005.12. Gao S, Wang Q, Tian XH, Li HL, Shen YH, Xu XK, Wu GZ, Hu ZL, Zhang WD. J Ethnopharmacol. 2017; 196:39–46.13. Engström MT, Pälijärvi M, Salminen JP. J Agric Food Chem. 2015; 63:4068–4079.14. Ahn KS, Noh EJ, Zhao HL, Jung SH, Kang SS, Kim YS. Life Sci. 2005; 76:2315–2328.15. Kim SF, Huri DA, Snyder SH. Science. 2005; 310:1966–1970.16. Tang X, Liu D, Shishodia S, Ozburn N, Behrens C, Lee JJ, Hong WK, Aggarwal BB, Wistuba II. Cancer. 2006; 107:2637–2646.17. Morris CJ. Methods Mol Biol. 2003; 225:115–121.18. Guo C, Zhang S, Teng S, Niu K. J Sep Sci. 2014; 37:950–956.19. Calixto JB. Braz J Med Biol Res. 2000; 33:179–189.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-inflammatory effects of proanthocyanidin-rich red rice extract via suppression of MAPK, AP-1 and NF-κB pathways in Raw 264.7 macrophages
- Luteolin 5-O-glucoside from Korean Milk Thistle, Cirsium maackii, Exhibits Anti-Inflammatory Activity via Activation of the Nrf2/HO-1 Pathway
- Anti-inflammatory activities of Scolopendra subspinipes mutilans in RAW 264.7 cells
- Rhodanthpyrone A and B play an anti-inflammatory role by suppressing the nuclear factor-κB pathway in macrophages
- Estragole Exhibits Anti-inflammatory Activity with the Regulation of NF-κB and Nrf-2 Signaling Pathways in LPS-induced RAW 264.7 cells