Nat Prod Sci.
2019 Mar;25(1):11-15. 10.20307/nps.2019.25.1.11.
Chemical Constituents of Bark of Beilschmiedia kunstleri Gamble with Anticancer, Anti-Streptococcus pyogenes, Anti-Bacillus cereus and Anti Plesiomonas shigelloides Activities
- Affiliations
-
- 1Faculty of Science, Qom University of Technology, Qom, Iran. a_mollataghi2@yahoo.com
- 2Department of Chemistry, Faculty of Science, University of Malaya, Kuala Lumpur 50603, Malaysia.
- KMID: 2443097
- DOI: http://doi.org/10.20307/nps.2019.25.1.11
Abstract
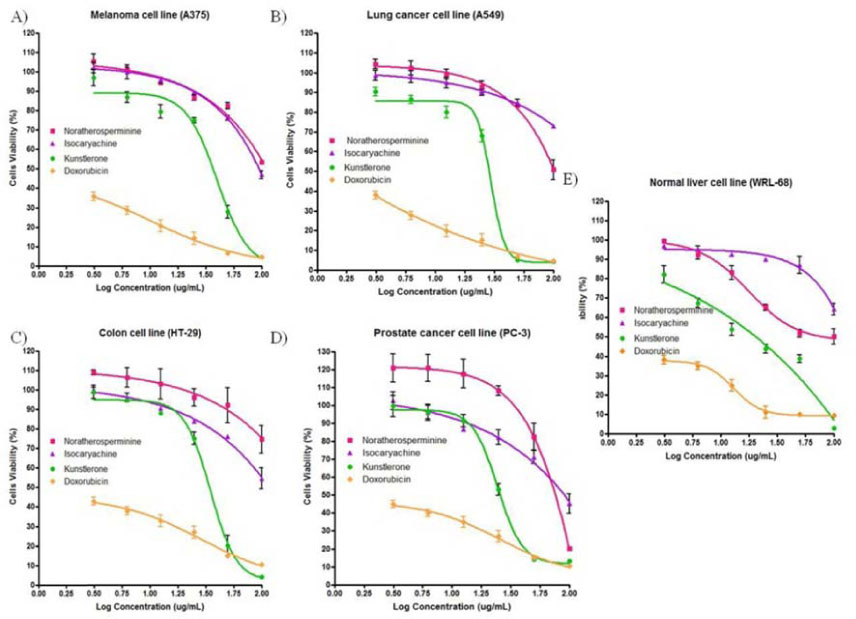
- Lauraceae is a family medicinal plant whose tubers possesses antimicrobial, and cytotoxic, such as antiparasitic and anti-inflammatory special effects and has been used for the medicine in the cure of hepatitis and rheumatism. The antimicrobial activities of bioactive compounds including one neolignan; kunstlerone (1) and two alkaloids include isocaryachine (2) and noratherosperminine (3) as well as crude hexane, methanol and dichloromethane extracts were evaluated. Additionally, the effect of compounds 1, 2 and 3 were evaluated on A549, PC-3, A375, HT-29 and WRL-68 cell lines. In conclusion, kunstlerone 1 showed moderate cytotoxicity against various cancer cell lines such as A549, PC-3, A375, HT-29 and WRL-68, respectively with ECâ‚…â‚€ values of 28.02, 26.78, 33.78, 33.65 and 16.46 µg/mL. The crude methanol extract showed antigrowth activity against S. pyogenes II and B. cereus, with MICs of 256 µg/mL. The compounds kunstlerone (1), isocaryachine (2) and noratherosperminine (3) showed complete inhibition against P. shigelloides, with MIC ≤60 µg/mL compare to ampicillin, as a positive control, which showed antigrowth activity against P. shigelloides at MIC 10 µg/mL.
Keyword
MeSH Terms
Figure
Reference
-
1. Simi A, Sokovi MD, Risti M, Gruji-Jovanovi S, Vukojevi J, Marin PD. Phytother Res. 2004; 18:713–717.2. Pudjiastuti P, Mukhtar MR, Hadi AH, Saidi N, Morita H, Litaudon M, Awang K. Molecules. 2010; 15:2339–2346.3. Lenta BN, Tantangmo F, Devkota KP, Wansi JD, Chouna JR, Soh RC, Neumann B, Stammler HG, Tsamo E, Sewald N. J Nat Prod. 2009; 72:2130–2134.4. Earl EA, Altaf M, Murikoli RV, Swift S, O'Toole R. BMC Complement Altern Med. 2010; 10:25.5. Lee KG, Shibamoto T. J Agric Food Chem. 2002; 50:4947–4952.6. Khan A, Safdar M, Ali Khan MM, Khattak KN, Anderson RA. Diabetes Care. 2003; 26:3215–3218.7. Saxton JE, Bentley KW. The Alkaloids: The isoquinoline alkaloids. The chemical Society;2007.8. Lajis NHJ, Ahmad R. Stud Nat Prod Chem. 2006; 33:1057–1090.9. Guinaudeau H, Leboeuf M, Cave A. J Nat Prod. 1979; 42:325–360.10. Harborne JB, Mendez J. Phytochemistry. 1969; 8:763–764.11. Yang PS, Cheng MJ, Peng CF, Chen JJ, Chen IS. J Nat Prod. 2009; 72:53–58.12. Yang PS, Cheng MJ, Chen JJ, Chen IS. Helv Chim Acta. 2008; 91:2130–2138.13. Sovová H, Opletal L, Bártlová M, Sajfrtová M, Krenková M. The Journal of supercritical fluids. 2007; 42:88–95.14. Jackson DE, Dewick PM. Phytochemistry. 1984; 23:1029–1035.15. Funasaki M, Lordello ALL, Viana AM, Santa-Catarina C, Floh EIS, Yoshida M, Kato MJ. J Braz Chem Soc. 2009; 20:853–859.16. Chouna JR, Nkeng-Efouet PA, Lenta BN, Devkota KP, Neumann B, Stammler HG, Kimbu SF, Sewald N. Phytochemistry. 2009; 70:684–688.17. Engler TA, Wei D, Letavic MA. Tetrahedron Lett. 1993; 34:1429–1432.18. Engler TA, Wei DD, Letavic MA, Combrink KD, Reddy JP. J Org Chem. 1994; 59:6588–6599.19. Lee JC, Kim HR, Kim J, Jang YS. J Agric Food Chem. 2002; 50:6490–6496.20. Braga PA, Dos Santos DA, De Silva MF, Vieira PC, Fernandes JB, Houghton PJ, Fang R. Nat Prod Res. 2007; 21:47–55.21. Mollataghi A, Hadi AHA, Awang K, Mohamad J, Litaudon M, Mukhtar MR. Molecules. 2011; 16:6582–6590.22. Mollataghi A, Hadi AHA, Cheah SC. Molecules. 2012; 17:4197–4208.23. Okusa P, Penge O, Devleeschouwer M, Duez P. J Ethnopharmacol. 2007; 112:476–481.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Traveler's Diarrhea by Plesiomonas shigelloides
- Two Cases of Diarrheal Disease Caused by Plesiomonas shigelloides
- Anticholinesterase and Anti-inflammatory Constituents from Beilschmiedia pulverulenta Kosterm
- Distributions of Bacillus cereus, Pseudomonas, Enterococcus, and coliforms Isolated from Agricultural Products
- A Case of Septicemia by Plesiomonas shigelloides