Diabetes Metab J.
2019 Apr;43(2):192-205. 10.4093/dmj.2018.0052.
Myricetin Protects Against High Glucose-Induced β-Cell Apoptosis by Attenuating Endoplasmic Reticulum Stress via Inactivation of Cyclin-Dependent Kinase 5
- Affiliations
-
- 1Department of Biomedical Science, Graduate School of Medicine, Kyungpook National University, Daegu, Korea.
- 2Department of Internal Medicine, Yeungnam University College of Medicine, Daegu, Korea.
- 3Institute of Medical Science, Yeungnam University College of Medicine, Daegu, Korea.
- 4Department of Internal Medicine, School of Medicine, Kyungpook National University, Kyungpook National University Hospital, Daegu, Korea. leei@knu.ac.kr
- 5New Drug Development Center, Daegu-Gyeongbuk Medical Innovation Foundation, Daegu, Korea.
- 6Department of Immunology, School of Medicine, Catholic University of Daegu, Daegu, Korea. jcim@cu.ac.kr
- KMID: 2442772
- DOI: http://doi.org/10.4093/dmj.2018.0052
Abstract
- BACKGROUND
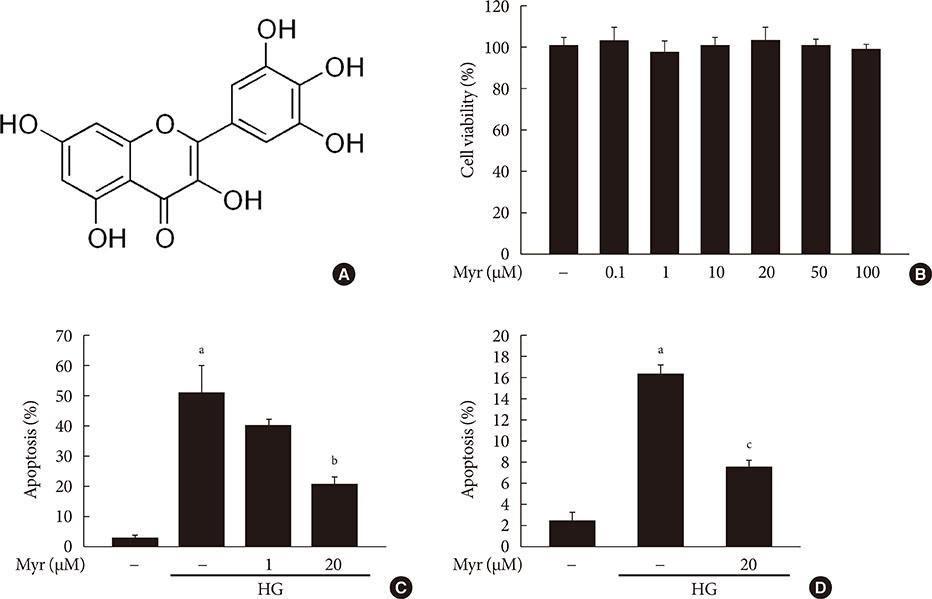
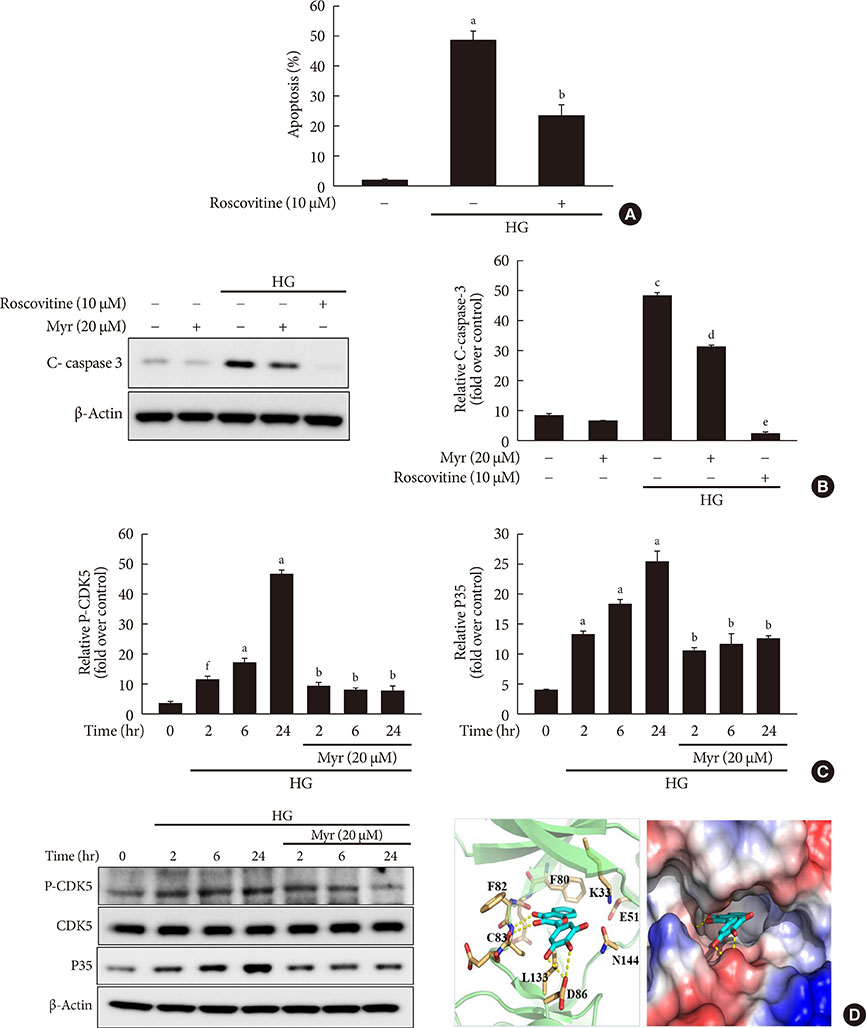
Chronic hyperglycemia has deleterious effects on pancreatic β-cell function and turnover. Recent studies support the view that cyclin-dependent kinase 5 (CDK5) plays a role in β-cell failure under hyperglycemic conditions. However, little is known about how CDK5 impair β-cell function. Myricetin, a natural flavonoid, has therapeutic potential for the treatment of type 2 diabetes mellitus. In this study, we examined the effect of myricetin on high glucose (HG)-induced β-cell apoptosis and explored the relationship between myricetin and CDK5.
METHODS
To address this question, we subjected INS-1 cells and isolated rat islets to HG conditions (30 mM) in the presence or absence of myricetin. Docking studies were conducted to validate the interaction between myricetin and CDK5. Gene expression and protein levels of endoplasmic reticulum (ER) stress markers were measured by real-time reverse transcription polymerase chain reaction and Western blot analysis.
RESULTS
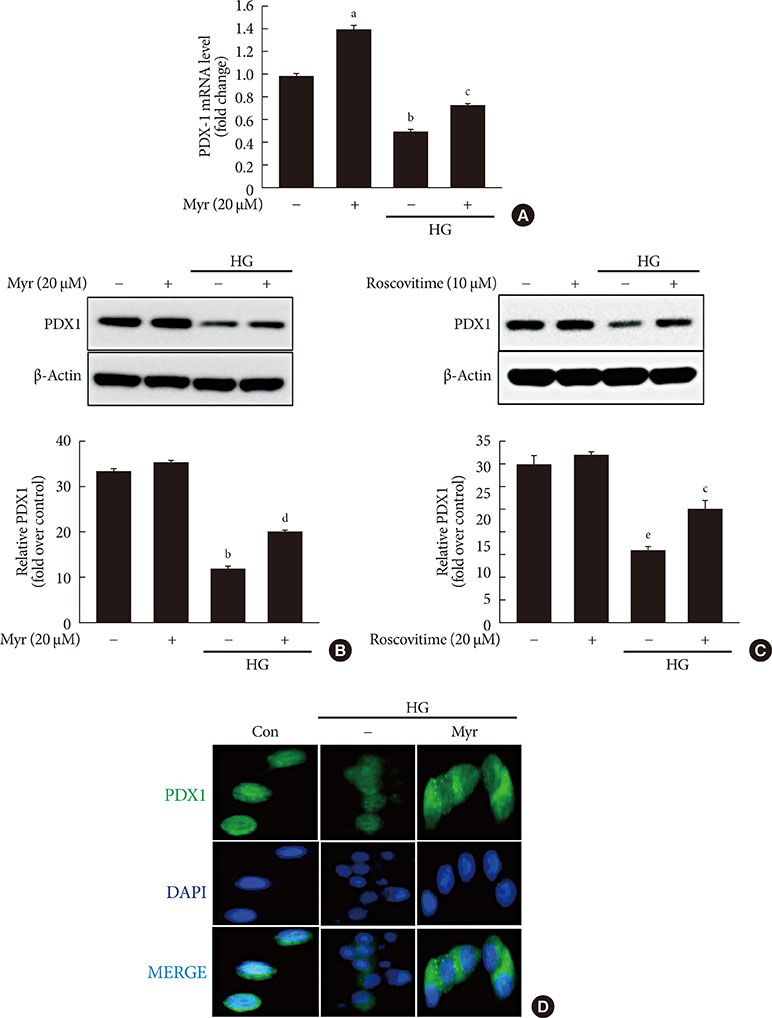
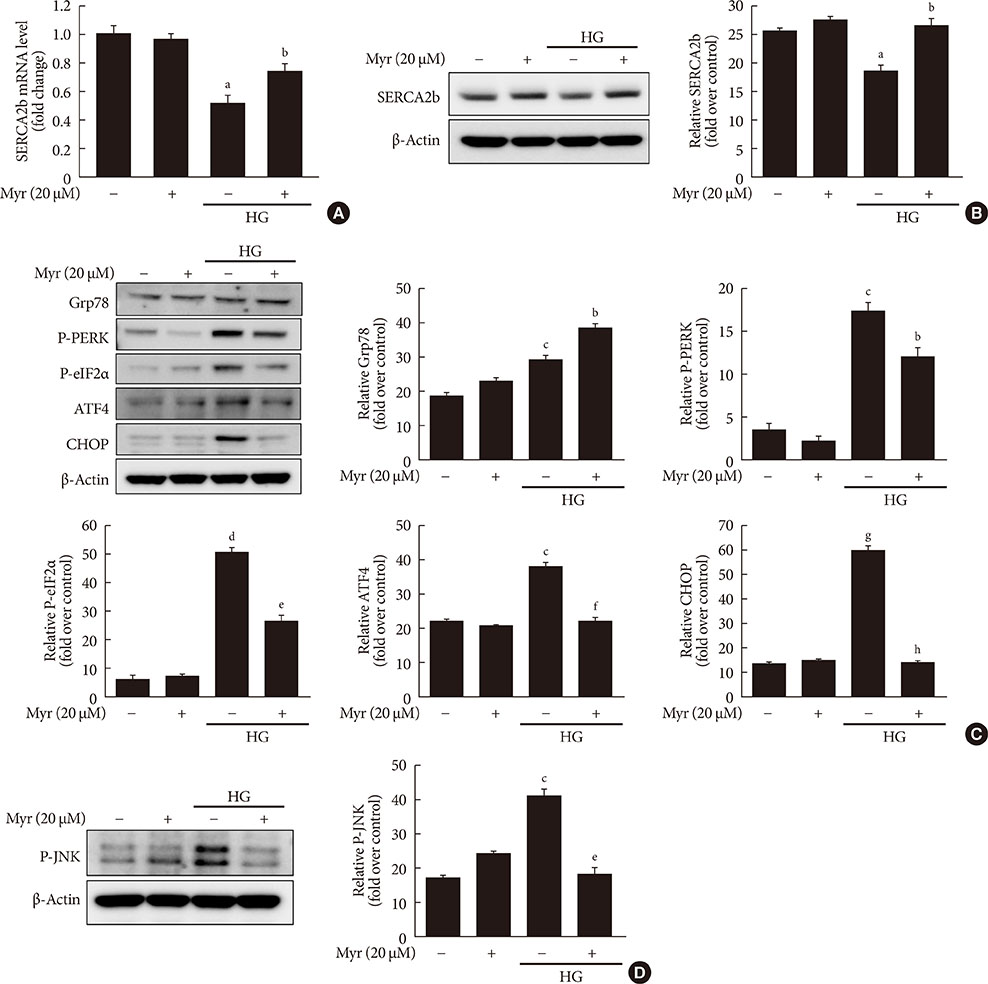
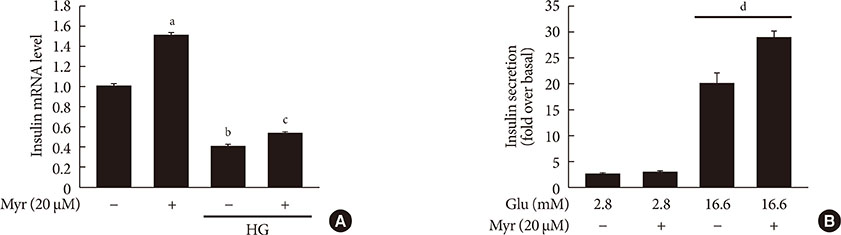
Activation of CDK5 in response to HG coupled with the induction of ER stress via the down regulation of sarcoendoplasmic reticulum calcium ATPase 2b (SERCA2b) gene expression and reduced the nuclear accumulation of pancreatic duodenal homeobox 1 (PDX1) leads to β-cell apoptosis. Docking study predicts that myricetin inhibit CDK5 activation by direct binding in the ATP-binding pocket. Myricetin counteracted the decrease in the levels of PDX1 and SERCA2b by HG. Moreover, myricetin attenuated HG-induced apoptosis in INS-1 cells and rat islets and reduce the mitochondrial dysfunction by decreasing reactive oxygen species production and mitochondrial membrane potential (Δψm) loss.
CONCLUSION
Myricetin protects the β-cells against HG-induced apoptosis by inhibiting ER stress, possibly through inactivation of CDK5 and consequent upregulation of PDX1 and SERCA2b.
Keyword
MeSH Terms
-
Animals
Apoptosis*
Blotting, Western
Calcium-Transporting ATPases
Cyclin-Dependent Kinase 5*
Diabetes Mellitus, Type 2
Down-Regulation
Endoplasmic Reticulum Stress*
Endoplasmic Reticulum*
Gene Expression
Genes, Homeobox
Glucose
Hyperglycemia
Insulin-Secreting Cells
Membrane Potential, Mitochondrial
Polymerase Chain Reaction
Rats
Reactive Oxygen Species
Reticulum
Reverse Transcription
Up-Regulation
Calcium-Transporting ATPases
Cyclin-Dependent Kinase 5
Glucose
Reactive Oxygen Species
Figure
Reference
-
1. Leahy JL. Pathogenesis of type 2 diabetes mellitus. Arch Med Res. 2005; 36:197–209.2. Poitout V, Robertson RP. Minireview: secondary beta-cell failure in type 2 diabetes. A convergence of glucotoxicity and lipotoxicity. Endocrinology. 2002; 143:339–342.3. Fonseca SG, Gromada J, Urano F. Endoplasmic reticulum stress and pancreatic β-cell death. Trends Endocrinol Metab. 2011; 22:266–274.4. Karunakaran U, Kim HJ, Kim JY, Lee IK. Guards and culprits in the endoplasmic reticulum: glucolipotoxicity and β-cell failure in type II diabetes. Exp Diabetes Res. 2012; 2012:639762.5. Hara T, Mahadevan J, Kanekura K, Hara M, Lu S, Urano F. Calcium efflux from the endoplasmic reticulum leads to β-cell death. Endocrinology. 2014; 155:758–768.6. Wei FY, Tomizawa K. Cyclin-dependent kinase 5 (Cdk5): a potential therapeutic target for the treatment of neurodegenerative diseases and diabetes mellitus. Mini Rev Med Chem. 2007; 7:1070–1074.7. Weishaupt JH, Kussmaul L, Grotsch P, Heckel A, Rohde G, Romig H, Bahr M, Gillardon F. Inhibition of CDK5 is protective in necrotic and apoptotic paradigms of neuronal cell death and prevents mitochondrial dysfunction. Mol Cell Neurosci. 2003; 24:489–502.8. Sun KH, de Pablo Y, Vincent F, Shah K. Deregulated Cdk5 promotes oxidative stress and mitochondrial dysfunction. J Neurochem. 2008; 107:265–278.9. Ubeda M, Kemp DM, Habener JF. Glucose-induced expression of the cyclin-dependent protein kinase 5 activator p35 involved in Alzheimer’s disease regulates insulin gene transcription in pancreatic beta-cells. Endocrinology. 2004; 145:3023–3031.10. Ubeda M, Rukstalis JM, Habener JF. Inhibition of cyclin-dependent kinase 5 activity protects pancreatic beta cells from glucotoxicity. J Biol Chem. 2006; 281:28858–28864.11. Wei FY, Nagashima K, Ohshima T, Saheki Y, Lu YF, Matsushita M, Yamada Y, Mikoshiba K, Seino Y, Matsui H, Tomizawa K. Cdk5-dependent regulation of glucose-stimulated insulin secretion. Nat Med. 2005; 11:1104–1108.12. Zheng YL, Hu YF, Zhang A, Wang W, Li B, Amin N, Grant P, Pant HC. Overexpression of p35 in Min6 pancreatic beta cells induces a stressed neuron-like apoptosis. J Neurol Sci. 2010; 299:101–107.13. Semwal DK, Semwal RB, Combrinck S, Viljoen A. Myricetin: a dietary molecule with diverse biological activities. Nutrients. 2016; 8:90.14. Liu IM, Tzeng TF, Liou SS, Lan TW. Myricetin, a naturally occurring flavonol, ameliorates insulin resistance induced by a high-fructose diet in rats. Life Sci. 2007; 81:1479–1488.15. Liu IM, Tzeng TF, Liou SS, Lan TW. Improvement of insulin sensitivity in obese Zucker rats by myricetin extracted from Abelmoschus moschatus. Planta Med. 2007; 73:1054–1060.16. Choi HN, Kang MJ, Lee SJ, Kim JI. Ameliorative effect of myricetin on insulin resistance in mice fed a high-fat, high-sucrose diet. Nutr Res Pract. 2014; 8:544–549.17. Ding Y, Zhang ZF, Dai XQ, Li Y. Myricetin protects against cytokine-induced cell death in RIN-m5f β cells. J Med Food. 2012; 15:733–740.18. Park KG, Lee KM, Seo HY, Suh JH, Kim HS, Wang L, Won KC, Lee HW, Park JY, Lee KU, Kim JG, Kim BW, Choi HS, Lee IK. Glucotoxicity in the INS-1 rat insulinoma cell line is mediated by the orphan nuclear receptor small heterodimer partner. Diabetes. 2007; 56:431–437.19. Sivitz WI, Yorek MA. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid Redox Signal. 2010; 12:537–577.20. Zapata-Torres G, Opazo F, Salgado C, Munoz JP, Krautwurst H, Mascayano C, Sepulveda-Boza S, Maccioni RB, Cassels BK. Effects of natural flavones and flavonols on the kinase activity of Cdk5. J Nat Prod. 2004; 67:416–420.21. Fujimoto K, Polonsky KS. Pdx1 and other factors that regulate pancreatic beta-cell survival. Diabetes Obes Metab. 2009; 11:Suppl 4. 30–37.22. Guo S, Dai C, Guo M, Taylor B, Harmon JS, Sander M, Robertson RP, Powers AC, Stein R. Inactivation of specific β cell transcription factors in type 2 diabetes. J Clin Invest. 2013; 123:3305–3316.23. Johnson JS, Kono T, Tong X, Yamamoto WR, Zarain-Herzberg A, Merrins MJ, Satin LS, Gilon P, Evans-Molina C. Pancreatic and duodenal homeobox protein 1 (Pdx-1) maintains endoplasmic reticulum calcium levels through transcriptional regulation of sarco-endoplasmic reticulum calcium ATPase 2b (SERCA2b) in the islet β cell. J Biol Chem. 2014; 289:32798–32810.24. Noroozi M, Burns J, Crozier A, Kelly IE, Lean ME. Prediction of dietary flavonol consumption from fasting plasma concentration or urinary excretion. Eur J Clin Nutr. 2000; 54:143–149.25. Kang BY, Kim SH, Cho D, Kim TS. Inhibition of interleukin-12 production in mouse macrophages via decreased nuclear factor-kappaB DNA binding activity by myricetin, a naturally occurring flavonoid. Arch Pharm Res. 2005; 28:274–279.26. Lee YS, Choi EM. Myricetin inhibits IL-1beta-induced inflammatory mediators in SW982 human synovial sarcoma cells. Int Immunopharmacol. 2010; 10:812–814.27. Zhang K, Ma Z, Wang J, Xie A, Xie J. Myricetin attenuated MPP(+)-induced cytotoxicity by anti-oxidation and inhibition of MKK4 and JNK activation in MES23.5 cells. Neuropharmacology. 2011; 61:329–335.28. Bhola PD, Letai A. Mitochondria-judges and executioners of cell death sentences. Mol Cell. 2016; 61:695–704.29. Zukerberg LR, Patrick GN, Nikolic M, Humbert S, Wu CL, Lanier LM, Gertler FB, Vidal M, Van Etten RA, Tsai LH. Cables links Cdk5 and c-Abl and facilitates Cdk5 tyrosine phosphorylation, kinase upregulation, and neurite outgrowth. Neuron. 2000; 26:633–646.30. Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008; 27:6407–6418.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endoplasmic Reticulum Stress Responses and Apoptosis
- Functional Inactivation of pRb Associated with Cyclin D1- and Cyclin-dependent Kinase 4 Overexpression Plays A Key Role in Human Pituitary Tumorigenesis
- Endoplasmic Reticulum Stress and Diabetes
- Puromycin aminonucleoside triggers apoptosis in podocytes by inducing endoplasmic reticulum stress
- Celecoxib induces cell death on non-small cell lung cancer cells through endoplasmic reticulum stress