Diabetes Metab J.
2019 Apr;43(2):174-182. 10.4093/dmj.2018.0046.
Discrepancies between Glycosylated Hemoglobin and Fasting Plasma Glucose for Diagnosing Impaired Fasting Glucose and Diabetes Mellitus in Korean Youth and Young Adults
- Affiliations
-
- 1Department of Pediatrics, Inje University Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Korea.
- 2Department of Pediatrics, Seoul National University Children's Hospital, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Pediatrics, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea. pedendo@snubh.org
- 4Department of Pediatrics, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2442770
- DOI: http://doi.org/10.4093/dmj.2018.0046
Abstract
- BACKGROUND
Glycosylated hemoglobin (HbA1c) has been recommended as a diagnostic test for prediabetes and diabetes. Here, we evaluated the level of agreement between diagnoses based on fasting plasma glucose (FPG) versus HbA1c levels and determined optimal HbA1c cutoff values for these diseases in youth and young adults.
METHODS
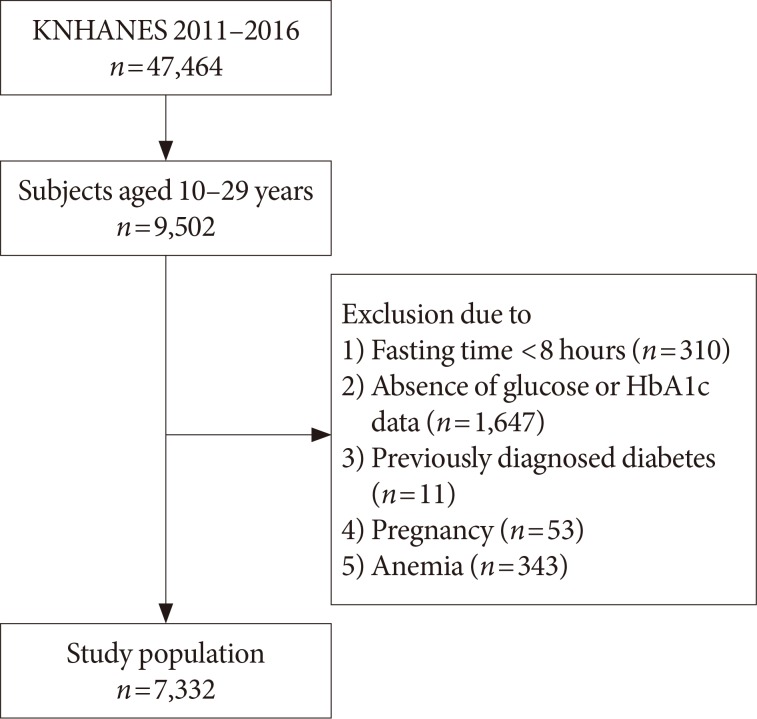
The study included 7,332 subjects (n=4,129, aged 10 to 19 years in youth group; and n=3,203 aged 20 to 29 years in young adult group) from the 2011 to 2016 Korea National Health and Nutrition Examination Survey. Prediabetes and diabetes were defined as 100 to 125 mg/dL (impaired fasting glucose [IFG]) and ≥126 mg/dL for FPG (diabetes mellitus [DM] by FPG [DMFPG]), and 5.7% to 6.4% and ≥6.5% for HbA1c, respectively.
RESULTS
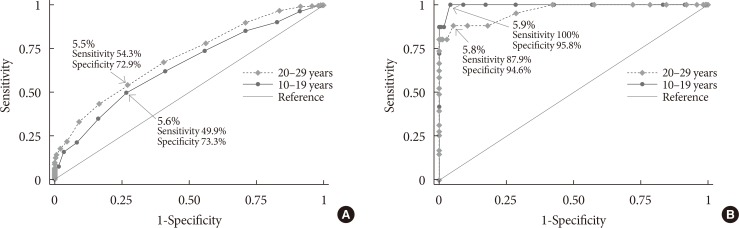
In the youth group, 32.5% with IFG had an HbA1c level of 5.7% to 6.4%, and 72.2% with DMFPG had an HbA1c ≥6.5%. In the young adult group, 27.5% with IFG had an HbA1c level of 5.7% to 6.4%, and 66.6% with DMFPG had an HbA1c ≥6.5%. Kappa coefficients for agreement between the FPG and HbA1c results were 0.12 for the youth group and 0.19 for the young adult group. In receiver operating characteristic curve analysis, the optimal HbA1c cutoff for IFG and DMFPG were 5.6% and 5.9% in youths and 5.5% and 5.8% in young adults, respectively.
CONCLUSION
Usefulness of HbA1c for diagnosis of IFG and DMFPG in Koreans aged <30 years remains to be determined due to discrepancies between the results of glucose- and HbA1c-based tests. Additional testing might be warranted at lower HbA1c levels to detect IFG and DMFPG in this age group.
Keyword
MeSH Terms
Figure
Reference
-
1. Cali AM, Caprio S. Prediabetes and type 2 diabetes in youth: an emerging epidemic disease? Curr Opin Endocrinol Diabetes Obes. 2008; 15:123–127. PMID: 18316946.
Article2. Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med. 2002; 19:708–723. PMID: 12207806.
Article3. Gerstein HC, Santaguida P, Raina P, Morrison KM, Balion C, Hunt D, Yazdi H, Booker L. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract. 2007; 78:305–312. PMID: 17601626.
Article4. Ek AE, Rossner SM, Hagman E, Marcus C. High prevalence of prediabetes in a Swedish cohort of severely obese children. Pediatr Diabetes. 2015; 16:117–128. PMID: 24635861.
Article5. Nguyen QM, Srinivasan SR, Xu JH, Chen W, Berenson GS. Fasting plasma glucose levels within the normoglycemic range in childhood as a predictor of prediabetes and type 2 diabetes in adulthood: the Bogalusa Heart Study. Arch Pediatr Adolesc Med. 2010; 164:124–128. PMID: 20124140.
Article6. International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009; 32:1327–1334. PMID: 19502545.7. American Diabetes Association. Standards of medical care in diabetes: 2010. Diabetes Care. 2010; 33(Suppl 1):S11–S61. PMID: 20042772.8. Ko SH, Kim SR, Kim DJ, Oh SJ, Lee HJ, Shim KH, Woo MH, Kim JY, Kim NH, Kim JT, Kim CH, Kim HJ, Jeong IK, Hong EK, Cho JH, Mok JO, Yoon KH; Committee of Clinical Practice Guidelines, Korean Diabetes Association. 2011 Clinical practice guidelines for type 2 diabetes in Korea. Diabetes Metab J. 2011; 35:431–436. PMID: 22111032.
Article9. Edelman D, Olsen MK, Dudley TK, Harris AC, Oddone EZ. Utility of hemoglobin A1c in predicting diabetes risk. J Gen Intern Med. 2004; 19:1175–1180. PMID: 15610327.
Article10. Pradhan AD, Rifai N, Buring JE, Ridker PM. Hemoglobin A1c predicts diabetes but not cardiovascular disease in nondiabetic women. Am J Med. 2007; 120:720–727. PMID: 17679132.
Article11. Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, Coresh J, Brancati FL. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010; 362:800–811. PMID: 20200384.
Article12. Bloomgarden ZT. A1C: recommendations, debates, and questions. Diabetes Care. 2009; 32:e141–e147. PMID: 19940210.
Article13. Cowie CC, Rust KF, Byrd-Holt DD, Gregg EW, Ford ES, Geiss LS, Bainbridge KE, Fradkin JE. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988-2006. Diabetes Care. 2010; 33:562–568. PMID: 20067953.
Article14. Mann DM, Carson AP, Shimbo D, Fonseca V, Fox CS, Muntner P. Impact of A1C screening criterion on the diagnosis of pre-diabetes among U.S. adults. Diabetes Care. 2010; 33:2190–2195. PMID: 20628087.
Article15. Jeon JY, Ko SH, Kwon HS, Kim NH, Kim JH, Kim CS, Song KH, Won JC, Lim S, Choi SH, Jang MJ, Kim Y, Oh K, Kim DJ, Cha BY. Prevalence of diabetes and prediabetes according to fasting plasma glucose and HbA1c. Diabetes Metab J. 2013; 37:349–357. PMID: 24199164.
Article16. Lee HS, Park HK, Hwang JS. HbA1c and glucose intolerance in obese children and adolescents. Diabet Med. 2012; 29:e102–e105. PMID: 22273110.17. Nam HK, Cho WK, Kim JH, Rhie YJ, Chung S, Lee KH, Suh BK. HbA1c cutoff for prediabetes and diabetes based on oral glucose tolerance test in obese children and adolescents. J Korean Med Sci. 2018; 33:e93. PMID: 29542302.
Article18. Seo JY, Hwang SS, Kim JH, Lee YA, Lee SY, Shin CH, Yang SW. Distribution of glycated haemoglobin and its determinants in Korean youth and young adults: a nationwide population-based study. Sci Rep. 2018; 8:1962. PMID: 29386645.
Article19. Saaddine JB, Fagot-Campagna A, Rolka D, Narayan KM, Geiss L, Eberhardt M, Flegal KM. Distribution of HbA(1c) levels for children and young adults in the U.S.: Third National Health and Nutrition Examination Survey. Diabetes Care. 2002; 25:1326–1330. PMID: 12145229.20. Shah S, Kublaoui BM, Oden JD, White PC. Screening for type 2 diabetes in obese youth. Pediatrics. 2009; 124:573–579. PMID: 19620188.
Article21. Lee JM, Wu EL, Tarini B, Herman WH, Yoon E. Diagnosis of diabetes using hemoglobin A1c: should recommendations in adults be extrapolated to adolescents? J Pediatr. 2011; 158:947–952. PMID: 21195416.
Article22. Nowicka P, Santoro N, Liu H, Lartaud D, Shaw MM, Goldberg R, Guandalini C, Savoye M, Rose P, Caprio S. Utility of hemoglobin A(1c) for diagnosing prediabetes and diabetes in obese children and adolescents. Diabetes Care. 2011; 34:1306–1311. PMID: 21515842.
Article23. Kweon S, Kim Y, Jang MJ, Kim Y, Kim K, Choi S, Chun C, Khang YH, Oh K. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int J Epidemiol. 2014; 43:69–77. PMID: 24585853.
Article24. Ministry of Health and Welfare. The Korea National Health and Nutritional Examination Survey. cited 2018 Oct 23. Available from: https://knhanes.cdc.go.kr/knhanes/main.do.25. World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity 2011 (WHO/NMH/NHD/MNM/11.1). cited 2018 Oct 23. Available from: http://www.who.int/vmnis/indicators/haemoglobin.pdf.26. Yang SE, Park CJ, Nah J, Min WK, Chi HS. Hematologic characteristics and hemoglobin fraction analysis by high performance liquid chromatogaphy in patients with hypochromic microcytosis: trials for detection of beta-thalassemia. Korean J Lab Med. 2005; 25:145–151.27. The National Glycohemoglobin Standardization Program. List of NGSP certified methods. cited 2018 Oct 23. Available from: http://www.ngsp.org/docs/methods.pdf.28. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977; 33:159–174. PMID: 843571.
Article29. Akobeng AK. Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Paediatr. 2007; 96:644–647. PMID: 17376185.
Article30. Lee H, Oh JY, Sung YA, Kim DJ, Kim SH, Kim SG, Moon S, Park IeB, Rhee EJ, Chung CH, Kim BJ, Ku BJ. Optimal hemoglobin A1C cutoff value for diagnosing type 2 diabetes mellitus in Korean adults. Diabetes Res Clin Pract. 2013; 99:231–236. PMID: 23541039.
Article31. Hu Y, Liu W, Chen Y, Zhang M, Wang L, Zhou H, Wu P, Teng X, Dong Y, Zhou Jw, Xu H, Zheng J, Li S, Tao T, Hu Y, Jia Y. Combined use of fasting plasma glucose and glycated hemoglobin A1c in the screening of diabetes and impaired glucose tolerance. Acta Diabetol. 2010; 47:231–236. PMID: 19760291.
Article32. Bennett CM, Guo M, Dharmage SC. HbA(1c) as a screening tool for detection of type 2 diabetes: a systematic review. Diabet Med. 2007; 24:333–343. PMID: 17367307.33. Hosking J, Metcalf BS, Jeffery AN, Streeter AJ, Voss LD, Wilkin TJ. Divergence between HbA1c and fasting glucose through childhood: implications for diagnosis of impaired fasting glucose (Early Bird 52). Pediatr Diabetes. 2014; 15:214–219. PMID: 25705748.34. Pani LN, Korenda L, Meigs JB, Driver C, Chamany S, Fox CS, Sullivan L, D'Agostino RB, Nathan DM. Effect of aging on A1C levels in individuals without diabetes: evidence from the Framingham Offspring Study and the National Health and Nutrition Examination Survey 2001-2004. Diabetes Care. 2008; 31:1991–1996. PMID: 18628569.35. Kim CH, Kim HK, Bae SJ, Park JY, Lee KU. Discordance between fasting glucose-based and hemoglobin A1c-based diagnosis of diabetes mellitus in Koreans. Diabetes Res Clin Pract. 2011; 91:e8–e10. PMID: 20970868.
Article36. Li J, Ma H, Na L, Jiang S, Lv L, Li G, Zhang W, Na G, Li Y, Sun C. Increased hemoglobin A1c threshold for prediabetes remarkably improving the agreement between A1c and oral glucose tolerance test criteria in obese population. J Clin Endocrinol Metab. 2015; 100:1997–2005. PMID: 25751104.
Article37. Herman WH, Ma Y, Uwaifo G, Haffner S, Kahn SE, Horton ES, Lachin JM, Montez MG, Brenneman T, Barrett-Connor E. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care. 2007; 30:2453–2457. PMID: 17536077.
Article38. Ziemer DC, Kolm P, Weintraub WS, Vaccarino V, Rhee MK, Twombly JG, Narayan KM, Koch DD, Phillips LS. Glucose-independent, black-white differences in hemoglobin A1c levels: a cross-sectional analysis of 2 studies. Ann Intern Med. 2010; 152:770–777. PMID: 20547905.39. Cohen RM, LeCaire TJ, Lindsell CJ, Smith EP, D'Alessio DJ. Relationship of prospective GHb to glycated serum proteins in incident diabetic retinopathy: implications of the glycation gap for mechanism of risk prediction. Diabetes Care. 2008; 31:151–153. PMID: 17909088.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Combination of Fasting Plasma Glucose and Glycosylated Hemoglobin as a Predictor for Type 2 Diabetes in Korean Adults (Korean Diabetes J 33(4):306-314, 2009)
- The Combination of Fasting Plasma Glucose and Glycosylated Hemoglobin as a Predictor for Type 2 Diabetes in Korean Adults (Korean Diabetes J 33(4):306-314, 2009)
- Prevalence and Risk Factors for Diabetes Mellitus and Impaired Fasting Glucose of Adults
- Role of HbA1c in the Screening of Diabetes Mellitus in a Korean Rural Community
- Update on the current modalities used to screen high risk youth for prediabetes and/or type 2 diabetes mellitus