J Cardiovasc Imaging.
2019 Apr;27(2):73-92. 10.4250/jcvi.2019.27.e17.
Role of Cardiac Computed Tomography in the Diagnosis of Left Ventricular Myocardial Diseases
- Affiliations
-
- 1Department of Radiology, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea. ksm9723@yahoo.co.kr
- 2Department of Radiology, Korea University Anam Hospital, Seoul, Korea.
- 3Department of Radiology, Research Institute of Radiological Science, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2442741
- DOI: http://doi.org/10.4250/jcvi.2019.27.e17
Abstract
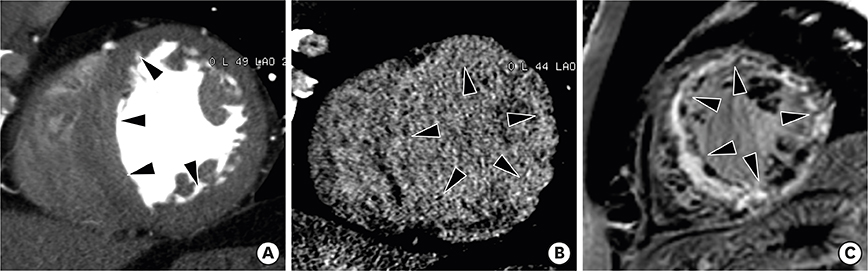
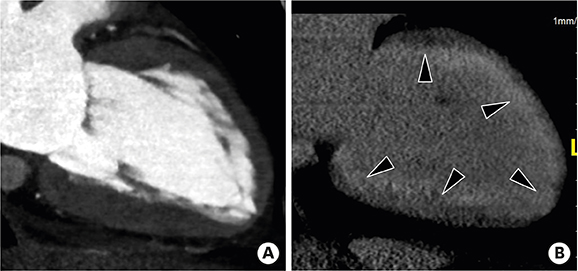
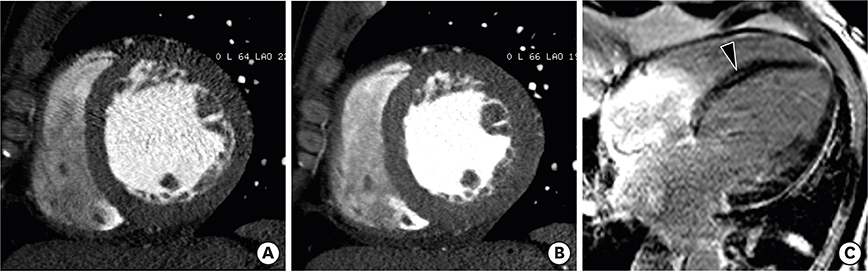
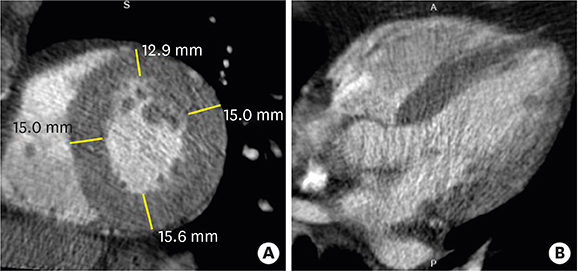
- Multimodality imaging is indicated for the evaluation of left ventricular (LV) myocardial diseases. Cardiac magnetic resonance (CMR) allows morphological and functional assessment of the LV along with soft tissue characterization. Technological advances in cardiac computed tomography (CT) have led to the development of techniques for diagnostic acquisition in LV myocardial disease. Cardiac CT facilitates the characterization of LV myocardial disease based on anatomy, function, and enhancement pattern. LV regional and global functional parameters are evaluated using multi-phasic cine CT images. CT myocardial perfusion facilitates the identification of hemodynamically significant coronary artery stenosis. Cardiac CT with delayed enhancement is used to detect myocardial scarring or fibrosis in myocardial infarction and non-ischemic cardiomyopathy, and for the measurement of extracellular volume fraction in non-ischemic cardiomyopathy. In this review, we review imaging techniques and key imaging features of cardiac CT used for the evaluation of myocardial diseases, along with CMR findings.
MeSH Terms
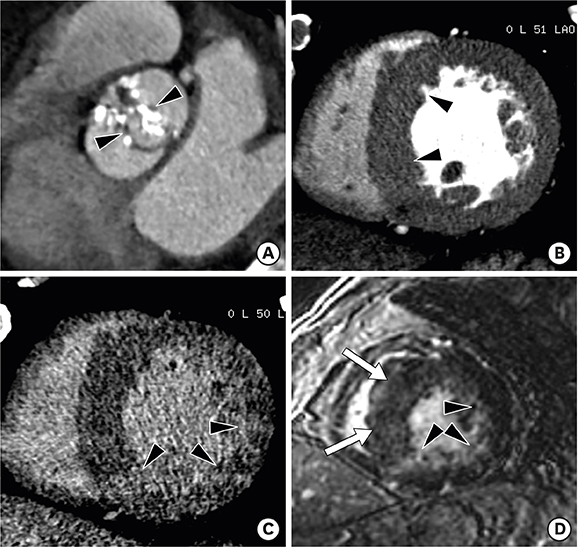
Figure
Cited by 1 articles
-
Simultaneous Assessment of Left Ventricular Function and Coronary Artery Anatomy by Third-generation Dual-source Computed Tomography Using a Low Radiation Dose
Ji Won Lee, Kyung Jin Nam, Jin You Kim, Yeon Joo Jeong, Geewon Lee, So Min Park, Soo Jin Lim, Ki Seok Choo
J Cardiovasc Imaging. 2020;28(1):21-32. doi: 10.4250/jcvi.2019.0066.
Reference
-
1. Nham E, Kim SM, Lee SC, et al. Association of cardiovascular disease risk factors with left ventricular mass, biventricular function, and the presence of silent myocardial infarction on cardiac MRI in an asymptomatic population. Int J Cardiovasc Imaging. 2016; 32:Suppl 1. 173–181.
Article2. Clayton B, Roobottom C, Morgan-Hughes G. Assessment of the myocardium with cardiac computed tomography. Eur Heart J Cardiovasc Imaging. 2014; 15:603–609.
Article3. Prasad K, Atherton J, Smith GC, McKenna WJ, Frenneaux MP, Nihoyannopoulos P. Echocardiographic pitfalls in the diagnosis of hypertrophic cardiomyopathy. Heart. 1999; 82:Suppl 3. III8–III15.
Article4. Chun EJ, Choi SI, Jin KN, et al. Hypertrophic cardiomyopathy: assessment with MR imaging and multidetector CT. Radiographics. 2010; 30:1309–1328.
Article5. Captur G, Manisty C, Moon JC. Cardiac MRI evaluation of myocardial disease. Heart. 2016; 102:1429–1435.
Article6. Machida H, Tanaka I, Fukui R, et al. Current and novel imaging techniques in coronary CT. Radiographics. 2015; 35:991–1010.
Article7. Treibel TA, Bandula S, Fontana M, et al. Extracellular volume quantification by dynamic equilibrium cardiac computed tomography in cardiac amyloidosis. J Cardiovasc Comput Tomogr. 2015; 9:585–592.
Article8. Kim YJ, Yong HS, Kim SM, Kim JA, Yang DH, Hong YJ. Korean guidelines for the appropriate use of cardiac CT. Korean J Radiol. 2015; 16:251–285.
Article9. Lewis MA, Pascoal A, Keevil SF, Lewis CA. Selecting a CT scanner for cardiac imaging: the heart of the matter. Br J Radiol. 2016; 89:20160376.
Article10. McCollough CH, Leng S, Yu L, Fletcher JG. Dual- and multi-energy CT: principles, technical approaches, and clinical applications. Radiology. 2015; 276:637–653.
Article11. Kalisz K, Halliburton S, Abbara S, et al. Update on cardiovascular applications of multienergy CT. Radiographics. 2017; 37:1955–1974.
Article12. Ko SM, Song MG, Chee HK, Hwang HK, Feuchtner GM, Min JK. Diagnostic performance of dual-energy CT stress myocardial perfusion imaging: direct comparison with cardiovascular MRI. AJR Am J Roentgenol. 2014; 203:W605-13.
Article13. Rossi A, Merkus D, Klotz E, Mollet N, de Feyter PJ, Krestin GP. Stress myocardial perfusion: imaging with multidetector CT. Radiology. 2014; 270:25–46.
Article14. Danad I, Szymonifka J, Schulman-Marcus J, Min JK. Static and dynamic assessment of myocardial perfusion by computed tomography. Eur Heart J Cardiovasc Imaging. 2016; 17:836–844.
Article15. Rossi A, Dharampal A, Wragg A, et al. Diagnostic performance of hyperaemic myocardial blood flow index obtained by dynamic computed tomography: does it predict functionally significant coronary lesions? Eur Heart J Cardiovasc Imaging. 2014; 15:85–94.
Article16. Ko SM, Kim YW, Han SW, Seo JB. Early and delayed myocardial enhancement in myocardial infarction using two-phase contrast-enhanced multidetector-row CT. Korean J Radiol. 2007; 8:94–102.
Article17. Lardo AC, Cordeiro MA, Silva C, et al. Contrast-enhanced multidetector computed tomography viability imaging after myocardial infarction: characterization of myocyte death, microvascular obstruction, and chronic scar. Circulation. 2006; 113:394–404.18. Mahnken AH, Koos R, Katoh M, et al. Assessment of myocardial viability in reperfused acute myocardial infarction using 16-slice computed tomography in comparison to magnetic resonance imaging. J Am Coll Cardiol. 2005; 45:2042–2047.
Article19. Gerber BL, Belge B, Legros GJ, et al. Characterization of acute and chronic myocardial infarcts by multidetector computed tomography: comparison with contrast-enhanced magnetic resonance. Circulation. 2006; 113:823–833.20. Bandula S, White SK, Flett AS, et al. Measurement of myocardial extracellular volume fraction by using equilibrium contrast-enhanced CT: validation against histologic findings. Radiology. 2013; 269:396–403.
Article21. Lee HJ, Im DJ, Youn JC, et al. Myocardial extracellular volume fraction with dual-energy equilibrium contrast-enhanced cardiac CT in nonischemic cardiomyopathy: a prospective comparison with cardiac MR imaging. Radiology. 2016; 280:49–57.
Article22. Abbara S, Blanke P, Maroules CD, et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: A report of the society of Cardiovascular Computed Tomography Guidelines Committee: Endorsed by the North American Society for Cardiovascular Imaging (NASCI). J Cardiovasc Comput Tomogr. 2016; 10:435–449.23. Asferg C, Usinger L, Kristensen TS, Abdulla J. Accuracy of multi-slice computed tomography for measurement of left ventricular ejection fraction compared with cardiac magnetic resonance imaging and two-dimensional transthoracic echocardiography: a systematic review and meta-analysis. Eur J Radiol. 2012; 81:e757–e762.24. Bak SH, Ko SM, Jeon HJ, Yang HS, Hwang HK, Song MG. Assessment of global left ventricular function with dual-source computed tomography in patients with valvular heart disease. Acta Radiol. 2012; 53:270–277.
Article25. Kang EJ, Lee KN, Choi WJ, et al. Left ventricular functional parameters and geometric patterns in Korean adults on coronary CT angiography with a 320-detector-row CT scanner. Korean J Radiol. 2017; 18:664–673.
Article26. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015; 16:233–270.
Article27. Roger VL, Go AS, Lloyd-Jones DM, et al. Executive summary: heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012; 125:188–197.28. Rajiah P, Desai MY, Kwon D, Flamm SD. MR imaging of myocardial infarction. Radiographics. 2013; 33:1383–1412.
Article29. Felker GM, Shaw LK, O'Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol. 2002; 39:210–218.
Article30. Kimura F, Matsuo Y, Nakajima T, et al. Myocardial fat at cardiac imaging: how can we differentiate pathologic from physiologic fatty infiltration? Radiographics. 2010; 30:1587–1602.
Article31. Zafar HM, Litt HI, Torigian DA. CT imaging features and frequency of left ventricular myocardial fat in patients with CT findings of chronic left ventricular myocardial infarction. Clin Radiol. 2008; 63:256–262.
Article32. Ichikawa Y, Kitagawa K, Chino S, et al. Adipose tissue detected by multislice computed tomography in patients after myocardial infarction. JACC Cardiovasc Imaging. 2009; 2:548–555.
Article33. La Grutta L, Toia P, Maffei E, Cademartiri F, Lagalla R, Midiri M. Infarct characterization using CT. Cardiovasc Diagn Ther. 2017; 7:171–188.
Article34. Vliegenthart R, Henzler T, Moscariello A, et al. CT of coronary heart disease: Part 1, CT of myocardial infarction, ischemia, and viability. AJR Am J Roentgenol. 2012; 198:531–547.
Article35. Cwajg JM, Cwajg E, Nagueh SF, et al. End-diastolic wall thickness as a predictor of recovery of function in myocardial hibernation: relation to rest-redistribution T1-201 tomography and dobutamine stress echocardiography. J Am Coll Cardiol. 2000; 35:1152–1161.36. Nieman K, Cury RC, Ferencik M, et al. Differentiation of recent and chronic myocardial infarction by cardiac computed tomography. Am J Cardiol. 2006; 98:303–308.
Article37. Rodriguez-Granillo GA. Delayed enhancement cardiac computed tomography for the assessment of myocardial infarction: from bench to bedside. Cardiovasc Diagn Ther. 2017; 7:159–170.
Article38. Sato A, Nozato T, Hikita H, et al. Prognostic value of myocardial contrast delayed enhancement with 64-slice multidetector computed tomography after acute myocardial infarction. J Am Coll Cardiol. 2012; 59:730–738.
Article39. Sharma A, Kumar S. Overview of left ventricular outpouchings on cardiac magnetic resonance imaging. Cardiovasc Diagn Ther. 2015; 5:464–470.40. Makkuni P, Kotler MN, Figueredo VM. Diverticular and aneurysmal structures of the left ventricle in adults: report of a case within the context of a literature review. Tex Heart Inst J. 2010; 37:699–705.41. Frances C, Romero A, Grady D. Left ventricular pseudoaneurysm. J Am Coll Cardiol. 1998; 32:557–561.
Article42. Sharma RK, Gore R, Rosen BD, Arbab-Zadeh A. Diagnosis of left ventricular pseudoaneurysm by cardiac CT angiography. J Cardiovasc Comput Tomogr. 2014; 8:246–247.
Article43. Cardim N, Galderisi M, Edvardsen T, et al. Role of multimodality cardiac imaging in the management of patients with hypertrophic cardiomyopathy: an expert consensus of the European Association of Cardiovascular Imaging Endorsed by the Saudi Heart Association. Eur Heart J Cardiovasc Imaging. 2015; 16:280.
Article44. Biagini E, Coccolo F, Ferlito M, et al. Dilated-hypokinetic evolution of hypertrophic cardiomyopathy: prevalence, incidence, risk factors, and prognostic implications in pediatric and adult patients. J Am Coll Cardiol. 2005; 46:1543–1550.45. Harris KM, Spirito P, Maron MS, et al. Prevalence, clinical profile, and significance of left ventricular remodeling in the end-stage phase of hypertrophic cardiomyopathy. Circulation. 2006; 114:216–225.
Article46. Olivotto I, Gistri R, Petrone P, Pedemonte E, Vargiu D, Cecchi F. Maximum left ventricular thickness and risk of sudden death in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2003; 41:315–321.
Article47. Patel AR, Kramer CM. Role of cardiac magnetic resonance in the diagnosis and prognosis of nonischemic cardiomyopathy. JACC Cardiovasc Imaging. 2017; 10:1180–1193.48. Kim SS, Ko SM, Choi SI, Choi BH, Stillman AE. Sudden cardiac death from structural heart diseases in adults: imaging findings with cardiovascular computed tomography and magnetic resonance. Int J Cardiovasc Imaging. 2016; 32:Suppl 1. 21–43.
Article49. Hashimura H, Kimura F, Ishibashi-Ueda H, et al. Radiologic-pathologic correlation of primary and secondary cardiomyopathies: MR imaging and histopathologic findings in hearts from autopsy and transplantation. Radiographics. 2017; 37:719–736.
Article50. Kalisz K, Rajiah P. Computed tomography of cardiomyopathies. Cardiovasc Diagn Ther. 2017; 7:539–556.
Article51. Blankstein R, Waller AH. Evaluation of known or suspected cardiac sarcoidosis. Circ Cardiovasc Imaging. 2016; 9:e000867.
Article52. Isobe M, Tezuka D. Isolated cardiac sarcoidosis: clinical characteristics, diagnosis and treatment. Int J Cardiol. 2015; 182:132–140.
Article53. Kusano KF, Satomi K. Diagnosis and treatment of cardiac sarcoidosis. Heart. 2016; 102:184–190.
Article54. Lee HJ, Im DJ, Youn JC, et al. Assessment of myocardial delayed enhancement with cardiac computed tomography in cardiomyopathies: a prospective comparison with delayed enhancement cardiac magnetic resonance imaging. Int J Cardiovasc Imaging. 2017; 33:577–584.
Article55. Czeyda-Pommersheim F, Hwang M, Chen SS, Strollo D, Fuhrman C, Bhalla S. Amyloidosis: modern cross-sectional imaging. Radiographics. 2015; 35:1381–1392.
Article56. Bhogal S, Ladia V, Sitwala P, et al. Cardiac amyloidosis: an updated review with emphasis on diagnosis and future directions. Curr Probl Cardiol. 2018; 43:10–34.
Article57. Deux JF, Mihalache CI, Legou F, et al. Noninvasive detection of cardiac amyloidosis using delayed enhanced MDCT: a pilot study. Eur Radiol. 2015; 25:2291–2297.
Article58. Dec GW, Fuster V. Idiopathic dilated cardiomyopathy. N Engl J Med. 1994; 331:1564–1575.
Article59. McCrohon JA, Moon JC, Prasad SK, et al. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003; 108:54–59.
Article60. Nanjo S, Yoshikawa K, Harada M, et al. Correlation between left ventricular diastolic function and ejection fraction in dilated cardiomyopathy using magnetic resonance imaging with late gadolinium enhancement. Circ J. 2009; 73:1939–1944.
Article61. Weiford BC, Subbarao VD, Mulhern KM. Noncompaction of the ventricular myocardium. Circulation. 2004; 109:2965–2971.
Article62. Ritter M, Oechslin E, Sütsch G, Attenhofer C, Schneider J, Jenni R. Isolated noncompaction of the myocardium in adults. Mayo Clin Proc. 1997; 72:26–31.
Article63. Freedom RM, Yoo SJ, Perrin D, Taylor G, Petersen S, Anderson RH. The morphological spectrum of ventricular noncompaction. Cardiol Young. 2005; 15:345–364.
Article64. Sidhu MS, Uthamalingam S, Ahmed W, et al. Defining left ventricular noncompaction using cardiac computed tomography. J Thorac Imaging. 2014; 29:60–66.
Article65. Melendez-Ramirez G, Castillo-Castellon F, Espinola-Zavaleta N, Meave A, Kimura-Hayama ET. Left ventricular noncompaction: a proposal of new diagnostic criteria by multidetector computed tomography. J Cardiovasc Comput Tomogr. 2012; 6:346–354.
Article66. Brunetti L, DeSantis ER. Treatment of viral myocarditis caused by coxsackievirus B. Am J Health Syst Pharm. 2008; 65:132–137.
Article67. Feldman AM, McNamara D. Myocarditis. N Engl J Med. 2000; 343:1388–1398.
Article68. Okura Y, Dec GW, Hare JM, et al. A clinical and histopathologic comparison of cardiac sarcoidosis and idiopathic giant cell myocarditis. J Am Coll Cardiol. 2003; 41:322–329.
Article69. Drory Y, Turetz Y, Hiss Y, et al. Sudden unexpected death in persons less than 40 years of age. Am J Cardiol. 1991; 68:1388–1392.70. Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol. 2009; 53:1475–1487.
Article71. Kindermann I, Barth C, Mahfoud F, et al. Update on myocarditis. J Am Coll Cardiol. 2012; 59:779–792.
Article72. Bouleti C, Baudry G, Iung B, et al. Usefulness of late iodine enhancement on spectral CT in acute myocarditis. JACC Cardiovasc Imaging. 2017; 10:826–827.
Article73. Tröbs M, Brand M, Achenbach S, Marwan M. Ultra-low dose comprehensive cardiac CT imaging in a patient with acute myocarditis. J Cardiovasc Comput Tomogr. 2014; 8:475–476.
Article74. Marwick TH, Gillebert TC, Aurigemma G, et al. Recommendations on the use of echocardiography in adult hypertension: a report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE). Eur Heart J Cardiovasc Imaging. 2015; 16:577–605.
Article75. Maceira AM, Mohiaddin RH. Cardiovascular magnetic resonance in systemic hypertension. J Cardiovasc Magn Reson. 2012; 14:28.
Article76. Rodrigues JC, Amadu AM, Dastidar AG, et al. Prevalence and predictors of asymmetric hypertensive heart disease: insights from cardiac and aortic function with cardiovascular magnetic resonance. Eur Heart J Cardiovasc Imaging. 2016; 17:1405–1413.
Article77. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006; 368:1005–1011.
Article78. Maganti K, Rigolin VH, Sarano ME, Bonow RO. Valvular heart disease: diagnosis and management. Mayo Clin Proc. 2010; 85:483–500.
Article79. Badiani S, van Zalen J, Treibel TA, Bhattacharyya S, Moon JC, Lloyd G. Aortic stenosis, a left ventricular disease: insights from advanced imaging. Curr Cardiol Rep. 2016; 18:80.
Article80. Dweck MR, Joshi S, Murigu T, et al. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol. 2011; 58:1271–1279.81. Nigri M, Azevedo CF, Rochitte CE, et al. Contrast-enhanced magnetic resonance imaging identifies focal regions of intramyocardial fibrosis in patients with severe aortic valve disease: Correlation with quantitative histopathology. Am Heart J. 2009; 157:361–368.
Article82. Weidemann F, Herrmann S, Störk S, et al. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation. 2009; 120:577–584.
Article83. Ko SM, Song MG, Hwang HK. Evaluation of the aortic and mitral valves with cardiac computed tomography and cardiac magnetic resonance imaging. Int J Cardiovasc Imaging. 2012; 28:Suppl 2. 109–127.
Article84. Song I, Ko SM, Yi JG, Chee HK, Kim JS. Differences in aortic valve and left ventricular parameters related to the severity of myocardial fibrosis in patients with severe aortic valve stenosis. PLoS One. 2017; 12:e0170939.
Article85. Gaudron PD, Liu D, Scholz F, et al. The septal bulge--an early echocardiographic sign in hypertensive heart disease. J Am Soc Hypertens. 2016; 10:70–80.
Article86. Kelshiker MA, Mayet J, Unsworth B, Okonko DO. Basal septal hypertrophy. Curr Cardiol Rev. 2013; 9:325–330.
Article87. Ranasinghe I, Ayoub C, Cheruvu C, Freedman SB, Yiannikas J. Isolated hypertrophy of the basal ventricular septum: characteristics of patients with and without outflow tract obstruction. Int J Cardiol. 2014; 173:487–493.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pulling Bowstring of Gated Myocardial SPECT
- Cardiac Strain Analysis Using Cine Magnetic Resonance Imaging and Computed Tomography
- Pattern Analysis of Left Ventricular Remodeling Using Cardiac Computed Tomography in Children with Congenital Heart Disease: Preliminary Results
- The Role of Cardiac MRI in the Diagnosis of Fabry Disease
- Assessment of Left Ventricular Myocardial Diseases with Cardiac Computed Tomography