Transl Clin Pharmacol.
2019 Mar;27(1):6-11. 10.12793/tcp.2019.27.1.6.
Digital therapeutics and clinical pharmacology
- Affiliations
-
- 1Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Bundang Hospital, Seongnam 13620, Korea. jychung@snubh.org
- KMID: 2442240
- DOI: http://doi.org/10.12793/tcp.2019.27.1.6
Abstract
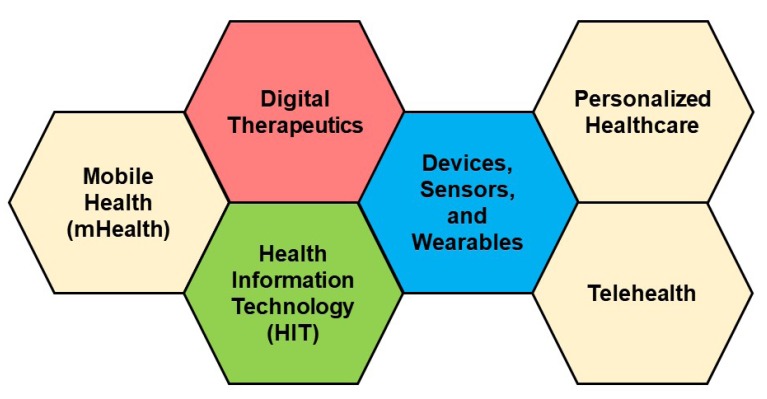
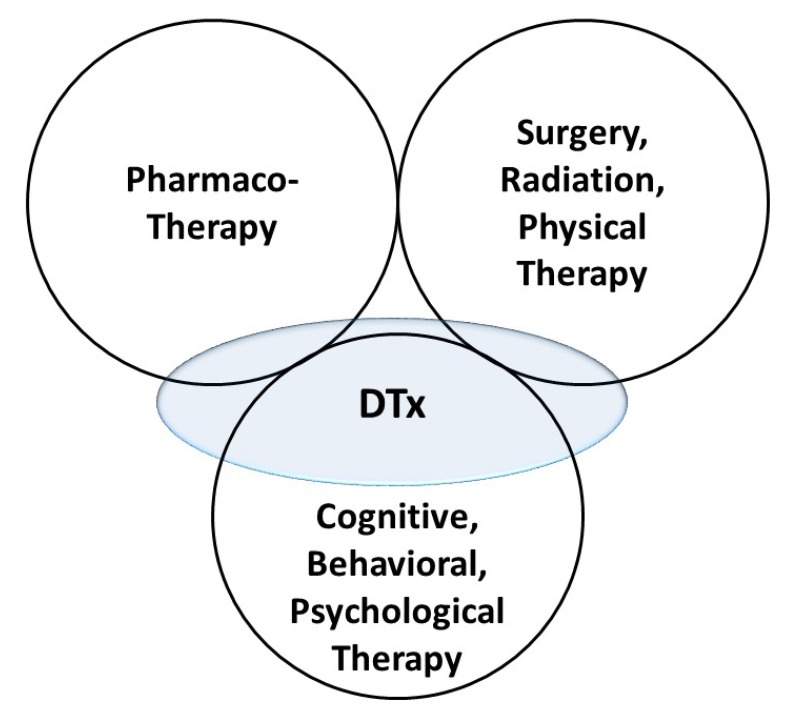
- Digital therapeutics (DTx) is a new subsection of digital health that is primarily driven by software and will be of great interest to clinical pharmacologists. In this article, an overview of DTx, including definition, position in the landscape of therapeutics, product categories, benefits, and challenges, is provided. Discussions from the point of view of clinical pharmacology are presented, as DTx should have exposure-response relationships. The principles of clinical pharmacology can be applied to DTx as they are comparable to pharmacotherapy. Clinical pharmacology has great potential in the development, application, and regulation of DTx.
Figure
Cited by 1 articles
-
Clinical Evaluation of Digital Therapeutics: Present and Future
Ki Young Huh, Jaeseong Oh, SeungHwan Lee, Kyung-Sang Yu
Healthc Inform Res. 2022;28(3):188-197. doi: 10.4258/hir.2022.28.3.188.
Reference
-
1. FDA permits marketing of mobile medical application for substance use disorder. Accessed 12 February 2019. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm576087.htm.2. Digital Therapeutics Alliance. Accessed 10 January 2019. https://www.dtxalliance.org/.3. Digital therapeutics. Accessed 10 January 2019. https://en.wikipedia.org/wiki/Digital_therapeutics.4. Digital Therapeutics: Combining Technology and Evidence-based Medicine to Transform Personalized Patient Care. Accessed 10 January 2019. https://www.dtxalliance.org/wp-content/uploads/2018/09/DTA-Report_DTx-Industry-Foundations.pdf.5. Plowman RS, Peters-Strickland T, Savage GM. Digital medicines: clinical review on the safety of tablets with sensors. Expert Opin Drug Saf. 2018; 17:849–852. DOI: 10.1080/14740338.2018.1508447. PMID: 30073875.6. Voelker R. Digital Pill Gains Approval. JAMA. 2018; 319:14. DOI: 10.1001/jama.2017.19309.
Article7. Examples of Mobile Apps For Which the FDA Will Exercise Enforcement Discretion. Accessed 12 February 2019. https://www.fda.gov/MedicalDevices/DigitalHealth/MobileMedicalApplications/ucm368744.htm.8. Pear Therapeutics receives Expedited Access Pathway designation from FDA for reSET-O™ prescription digital therapeutic to treat opioid use disorder. Accessed 10 January 2019. http://www.businesswire.com/news/home/20171018006174/en/Pear-Therapeutics-Receives-ExpeditedAccess-Pathway-Designation.9. Sverdlov O, van Dam J, Hannesdottir K, Thornton-Wells T. Digital Therapeutics: An Integral Component of Digital Innovation in Drug Development. Clin Pharmacol Ther. 2018; 104:72–80. DOI: 10.1002/cpt.1036. PMID: 29377057.
Article10. Luderer HF, Coolidge K, Campbell AN, Nunes EV, Maricich YA. reSET Digital Therapeutic for SUD Demonstrates Dose-Dependent Improvement in Outcomes. J Pharmacokinet Pharmacodyn. 2018; 45:S84–S85.11. Mattson MP, Calabrese EJ. Hormesis: what it is and why it matters. Hormesis. Springer;2010. p. 1–13.12. Lee TT, Kesselheim AS. U.S. Food and Drug Administration Precertification Pilot Program for Digital Health Software: Weighing the Benefits and Risks. Ann Intern Med. 2018; 168:730–732. DOI: 10.7326/M17-2715. PMID: 29632953.
Article