Investig Magn Reson Imaging.
2019 Mar;23(1):17-25. 10.13104/imri.2019.23.1.17.
Diagnostic Significance of pH-Responsive Gd³âº-Based Tâ‚ MR Contrast Agents
- Affiliations
-
- 1Bioimaging Research Team, Korea Basic Science Institute, Cheongju, Korea. kshong@kbsi.re.kr
- 2Amrita Centre for Industrial Research & Innovation, Amrita Vishwa Vidyapeetham, Ettimadai, Coimbatore, India.
- 3Graduate School of Analytical Science and Technology, Chungnam National University, Daejeon, Korea.
- KMID: 2442229
- DOI: http://doi.org/10.13104/imri.2019.23.1.17
Abstract
- We discuss recent advances in Gd-based Tâ‚-weighted MR contrast agents for the mapping of cellular pH. The pH plays a critical role in various biological processes. During the past two decades, several MR contrast agents of strategic importance for pH-mapping have been developed. Some of these agents shed light on the pH fluctuation in the tumor microenvironment. A pH-responsive self-assembled contrast agent facilitates the visualization of tumor size as small as 3 mm³. Optimization of various parameters is crucial for the development of pH-responsive contrast agents. In due course, the new contrast agents may provide significant insight into pH fluctuations in the human body.
Keyword
MeSH Terms
Figure
Reference
-
1. Boron WF, Boulpaep EL. Medical physiology: a cellular and molecular approach. Philadelphia: Saunders/Elsevier;2008.2. Lambers H, Piessens S, Bloem A, Pronk H, Finkel P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int J Cosmet Sci. 2006; 28:359–370.
Article3. Han J, Burgess K. Fluorescent indicators for intracellular pH. Chem Rev. 2010; 110:2709–2728.
Article4. Adrogué HJ, Wesson DE. Overview of acid base disorders. In : Adrogué HJ, Wesson DE, editors. Blackwell's basics of medicine. Acid-base. Boston: Blackwell Science;1994. p. 49–133.5. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009; 324:1029–1033.
Article6. Behne MJ, Barry NP, Hanson KM, et al. Neonatal development of the stratum corneum pH gradient: localization and mechanisms leading to emergence of optimal barrier function. J Invest Dermatol. 2003; 120:998–1006.
Article7. Ilic D, Mao-Qiang M, Crumrine D, et al. Focal adhesion kinase controls pH-dependent epidermal barrier homeostasis by regulating actin-directed Na+/H+ exchanger 1 plasma membrane localization. Am J Pathol. 2007; 170:2055–2067.
Article8. Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014; 157:832–844.
Article9. Lee MH, Park N, Yi C, et al. Mitochondria-immobilized pH-sensitive off-on fluorescent probe. J Am Chem Soc. 2014; 136:14136–14142.
Article10. Chen G, Fu Q, Yu F, et al. Wide-acidity-range pH fluorescence probes for evaluation of acidification in mitochondria and digestive tract mucosa. Anal Chem. 2017; 89:8509–8516.
Article11. Podder A, Won M, Kim S, et al. A two-photon fluorescent probe records the intracellular pH through ‘OR’ logic operation via internal calibration. Sens Actuators B Chem. 2018; 268:195–204.
Article12. Raghunand N, Altbach MI, van Sluis R, et al. Plasmalemmal pH-gradients in drug-sensitive and drug-resistant MCF-7 human breast carcinoma xenografts measured by 31P magnetic resonance spectroscopy. Biochem Pharmacol. 1999; 57:309–312.
Article13. Mason RP. Transmembrane pH gradients in vivo: measurements using fluorinated vitamin B6 derivatives. Curr Med Chem. 1999; 6:481–499.14. Ojugo AS, McSheehy PM, McIntyre DJ, et al. Measurement of the extracellular pH of solid tumours in mice by magnetic resonance spectroscopy: a comparison of exogenous (19)F and (31)P probes. NMR Biomed. 1999; 12:495–504.15. van Sluis R, Bhujwalla ZM, Raghunand N, et al. In vivo imaging of extracellular pH using 1H MRSI. Magn Reson Med. 1999; 41:743–750.16. Garcia-Martin ML, Herigault G, Remy C, et al. Mapping extracellular pH in rat brain gliomas in vivo by 1H magnetic resonance spectroscopic imaging: comparison with maps of metabolites. Cancer Res. 2001; 61:6524–6531.17. Vermathen P, Capizzano AA, Maudsley AA. Administration and (1)H MRS detection of histidine in human brain: application to in vivo pH measurement. Magn Reson Med. 2000; 43:665–675.18. Mori S, Eleff SM, Pilatus U, Mori N, van Zijl PC. Proton NMR spectroscopy of solvent-saturable resonances: a new approach to study pH effects in situ. Magn Reson Med. 1998; 40:36–42.19. Ward KM, Balaban RS. Determination of pH using water protons and chemical exchange dependent saturation transfer (CEST). Magn Reson Med. 2000; 44:799–802.
Article20. Goldman MR, Brady TJ, Pykett IL, et al. Quantification of experimental myocardial infarction using nuclear magnetic resonance imaging and paramagnetic ion contrast enhancement in excised canine hearts. Circulation. 1982; 66:1012–1016.
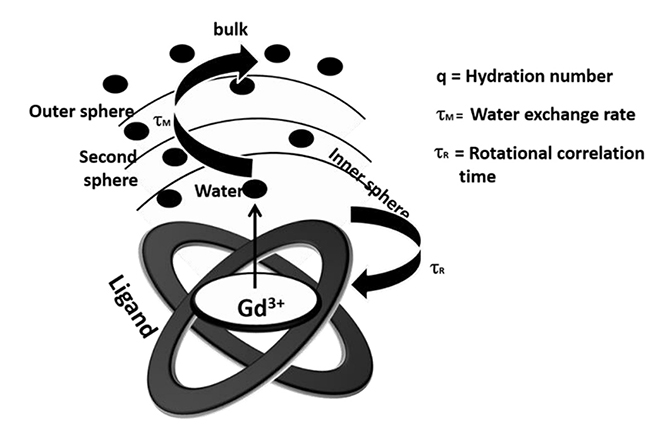
Article21. Caravan P. Strategies for increasing the sensitivity of gadolinium based MRI contrast agents. Chem Soc Rev. 2006; 35:512–523.
Article22. Koenig SH. A novel derivation of the Solomon-Bloembergen-Morgan equations: application to solvent relaxation by Mn2+-protein complexes. J Magn Reson. 1978; 31:1–10.23. Westlund PO. A generalized Solomon-Bloembergen-Morgan theory for arbitrary electron spin quantum number S - the dipole-dipole coupling between a nuclear spin I = 1/2 and an electron spin system S = 5/2. Mol Phys. 1995; 85:1165–1178.24. Kowalewski J, Luchinat C, Nilsson T, Parigi G. Nuclear spin relaxation in paramagnetic systems: electron spin relaxation effects under near-red field limit conditions and beyond. J Phys Chem A. 2002; 106:7376–7382.25. Yin J, Chen D, Zhang Y, Li C, Liu L, Shao Y. MRI relaxivity enhancement of gadolinium oxide nanoshells with a controllable shell thickness. Phys Chem Chem Phys. 2018; 20:10038–10047.
Article26. Zech SG, Eldredge HB, Lowe MP, Caravan P. Protein binding to lanthanide(III) complexes can reduce the water exchange rate at the lanthanide. Inorg Chem. 2007; 46:3576–3584.
Article27. Werner EJ, Datta A, Jocher CJ, Raymond KN. High-relaxivity MRI contrast agents: where coordination chemistry meets medical imaging. Angew Chem Int Ed Engl. 2008; 47:8568–8580.
Article28. Zhang S, Wu K, Sherry AD. A novel pH-Sensitive MRI contrast agent. Angew Chem Int Ed Engl. 1999; 38:3192–3194.
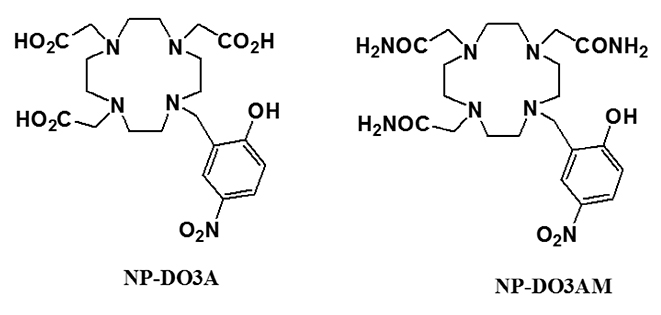
Article29. Ali MM, Woods M, Caravan P, et al. Synthesis and relaxometric studies of a dendrimer-based pH-responsive MRI contrast agent. Chemistry. 2008; 14:7250–7258.
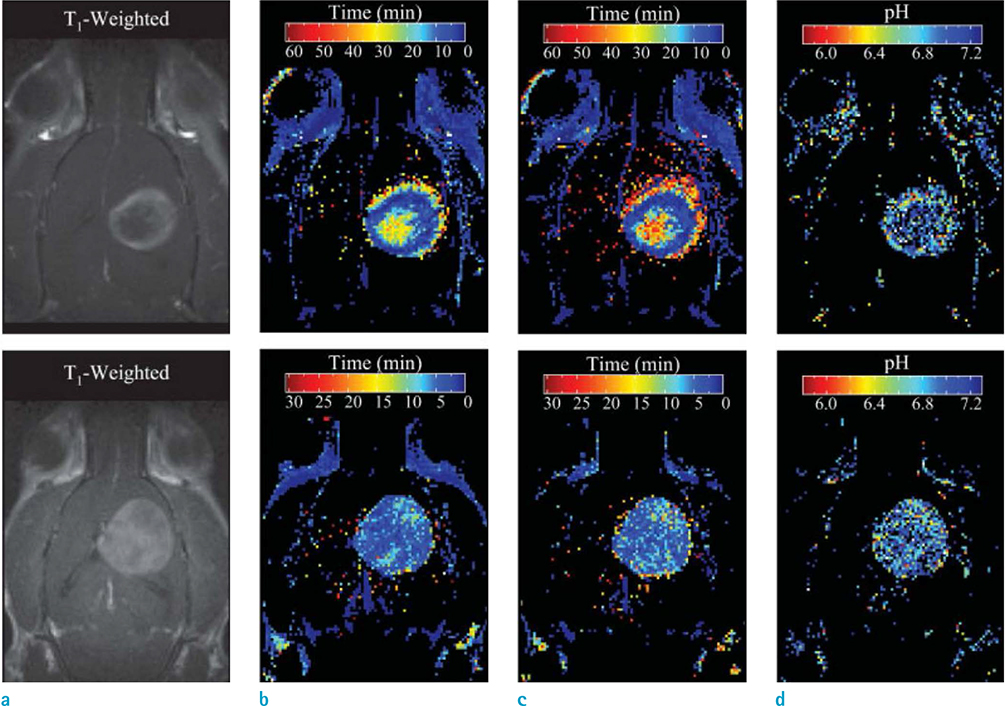
Article30. Garcia-Martin ML, Martinez GV, Raghunand N, Sherry AD, Zhang S, Gillies RJ. High resolution pH(e) imaging of rat glioma using pH-dependent relaxivity. Magn Reson Med. 2006; 55:309–315.
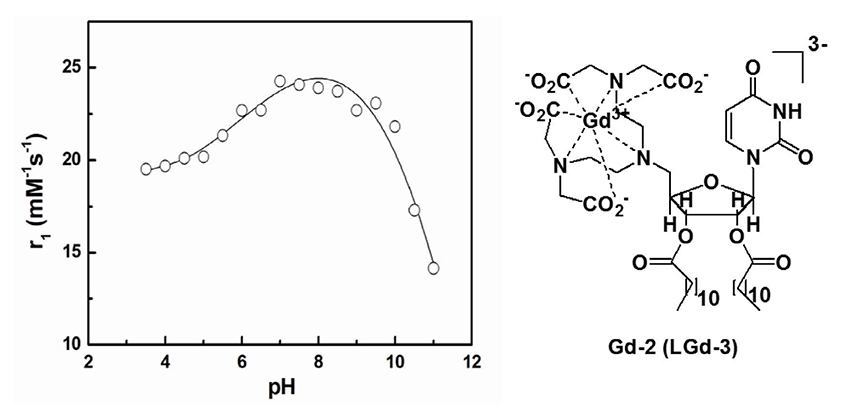
Article31. Aime S, Fedeli F, Sanino A, Terreno E. A R2/R1 ratiometric procedure for a concentration-independent, pH-responsive, Gd(III)-based MRI agent. J Am Chem Soc. 2006; 128:11326–11327.32. Toth E, Bolskar RD, Borel A, et al. Water-soluble gadofullerenes: toward high-relaxivity, pH-responsive MRI contrast agents. J Am Chem Soc. 2005; 127:799–805.33. Bhuniya S, Moon H, Lee H, et al. Uridine-based paramagnetic supramolecular nanoaggregate with high relaxivity capable of detecting primitive liver tumor lesions. Biomaterials. 2011; 32:6533–6540.
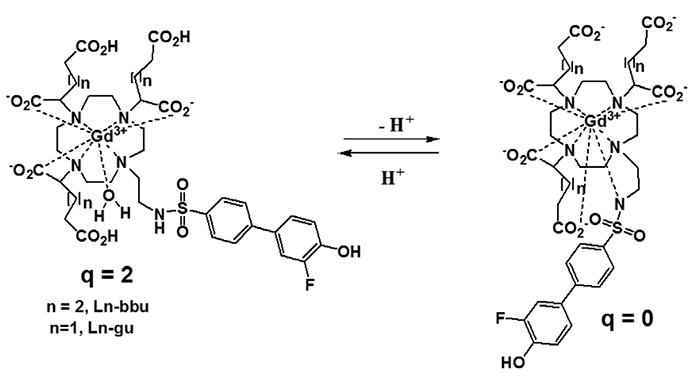
Article34. Woods M, Kiefer GE, Bott S, et al. Synthesis, relaxometric and photophysical properties of a new pH-responsive MRI contrast agent: the effect of other ligating groups on dissociation of a p-nitrophenolic pendant arm. J Am Chem Soc. 2004; 126:9248–9256.35. Frullano L, Catana C, Benner T, Sherry AD, Caravan P. Bimodal MR-PET agent for quantitative pH imaging. Angew Chem Int Ed Engl. 2010; 49:2382–2384.
Article36. Moriggi L, Yaseen MA, Helm L, Caravan P. Serum albumin targeted, pH-dependent magnetic resonance relaxation agents. Chemistry. 2012; 18:3675–3686.
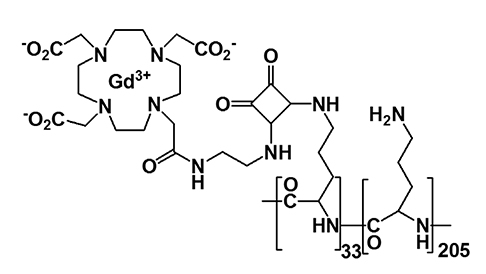
Article37. Kim KS, Park W, Hu J, Bae YH, Na K. A cancer-recognizable MRI contrast agents using pH-responsive polymeric micelle. Biomaterials. 2014; 35:337–343.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- MR Contrast Agents and Molecular Imaging
- Molecular MR Imaging
- Equilibrium Phase MR Angiography Using New Blood Pool Contrasts: Experimental Study in Rabbits
- The Significance of Perfusion Defect at Myocardial Perfusion MR Imaging in a Cat Model of Acute Reperfused Myocardial Infarction
- Safety of Administering Intravenous CT Contrast Agents Repeatedly or Using Both CT and MRI Contrast Agents on the Same Day: An Animal Study