J Rheum Dis.
2019 Jan;26(1):20-30. 10.4078/jrd.2019.26.1.20.
Effectiveness and Safety of Tacrolimus in Patients with Active Rheumatoid Arthritis with Inadequate Response to Disease-modifying Anti-rheumatic Drugs: The TREASURE Study
- Affiliations
-
- 1Division of Rheumatology, Eulji University Hospital, Daejeon, Korea.
- 2Division of Rheumatology, Kyung Hee University Medical Center, Seoul, Korea.
- 3Division of Rheumatology, Konkuk University Medical Center, Seoul, Korea.
- 4Division of Rheumatology, Hanyang University Guri Hospital, Guri, Korea.
- 5Division of Rheumatology, Dong-A University Hospital, Busan, Korea.
- 6Medical Affairs Asia Oceania, Astellas Pharma, Inc., Singapore.
- 7Medical Affairs, Astellas Pharma Korea, Inc., Seoul, Korea.
- 8Department of Rheumatology, Hanyang University Hospital for Rheumatic Diseases, Seoul, Korea. dhyoo@hanyang.ac.kr
- KMID: 2442031
- DOI: http://doi.org/10.4078/jrd.2019.26.1.20
Abstract
OBJECTIVE
Evaluate effectiveness/safety of tacrolimus in patients in Korea with active rheumatoid arthritis (RA) and unsuccessful response to disease-modifying anti-rheumatic drugs (DMARDs).
METHODS
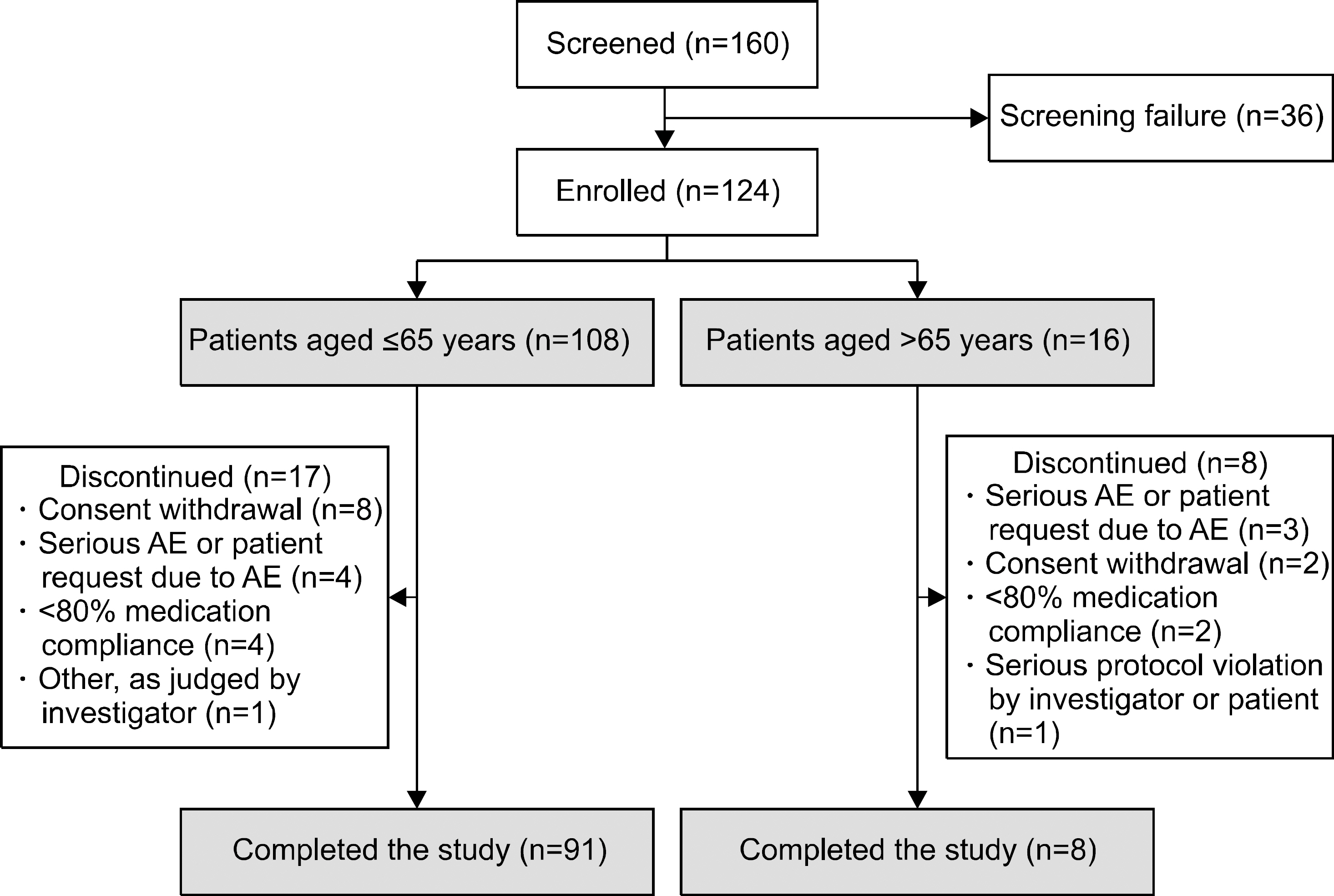
Open-label, single-arm, non-comparative, 24-week, Phase-IV study in patients with active RA who had taken DMARDs for >6 months. Following a washout period, tacrolimus was initiated (baseline-12 weeks; dose 2 mg/day and 1.5 mg/day in patients aged ≤65 and >65 years, respectively). After 12 weeks, dose could be adjusted (remaining between 1~3 mg); treatment continued to 24 weeks. Primary endpoint was American College of Rheumatology 20% improvement (ACR20) (baseline-Week 24). Secondary endpoints included ACR50/ACR70 response, disease-activity score in 28 joints (DAS28) erythrocyte sedimentation rate (ESR), number of tender/swollen joints, and bone mineral density (BMD) loss. Adverse events (AEs) were recorded.
RESULTS
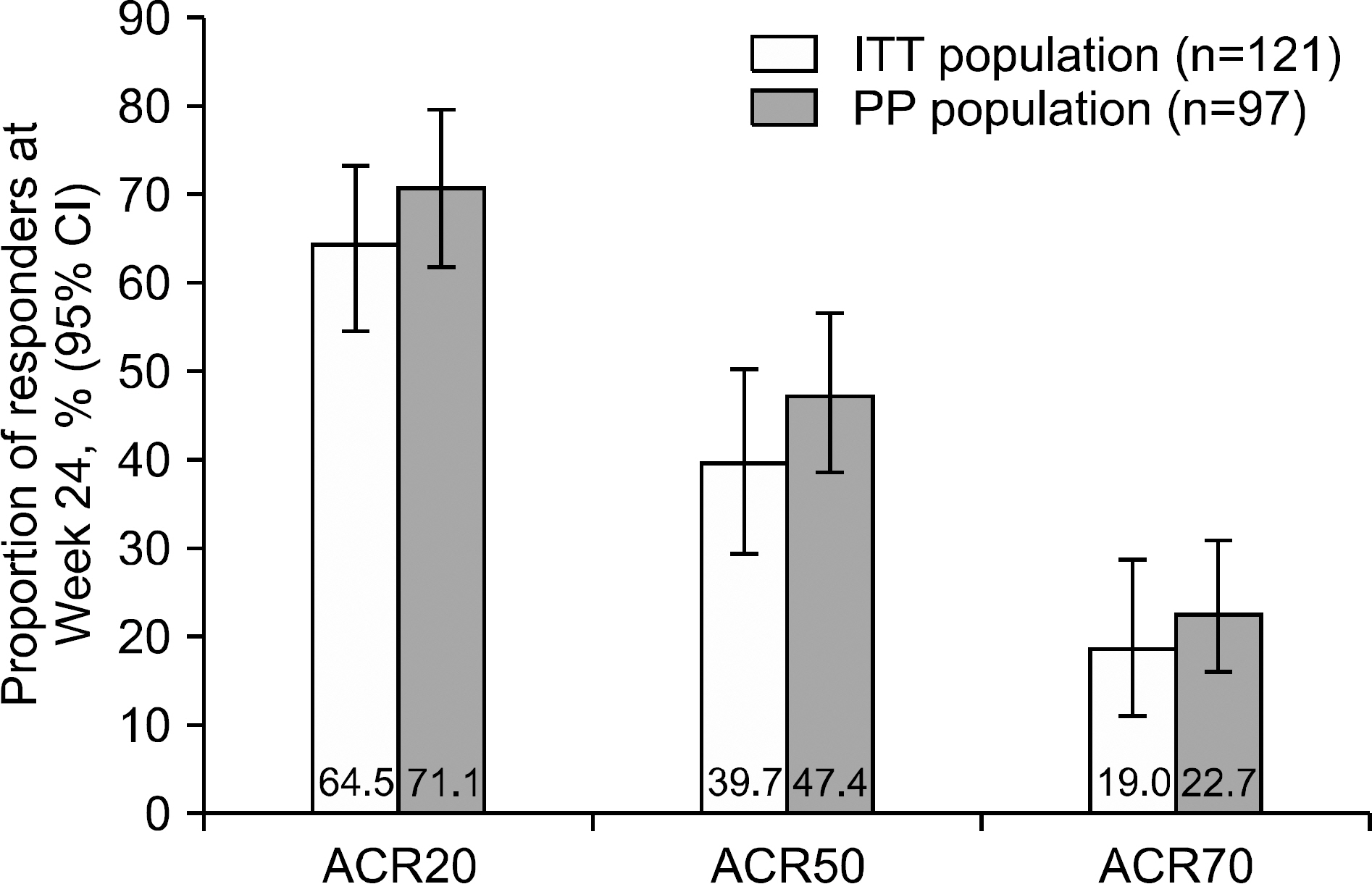
Overall, 121 patients were analysed. Mean±standard deviation tacrolimus dose baseline-Week 24 was 1.81±0.47 mg/day. After 24 weeks, 64.5%, 39.7%, and 19.0% of patients were ACR20, ACR50, and ACR70 responders, respectively. DAS28-ESR score decreased from 5.5±0.8 (baseline) to 3.7±1.5 (Week 24; p < 0.0001); number of tender/swollen joints decreased. Between screening and Week 24, change in BMD-T score in lumbar and femur regions was −0.06±0.38 (p=0.1550) and −0.04±0.28 (p=0.0936), respectively, with no significant change in International Society for Clinical Densitometry classification. Fifty-six (46.3%) patients experienced 93 AEs; 75.3% were mild. No unexpected safety signals identified.
CONCLUSION
Tacrolimus therapy was associated with a high proportion of ACR responders, and improved DAS28-ESR score and physical joint function during the study. Tacrolimus may be a suitable therapy for DMARD-resistant patients with RA.
MeSH Terms
Figure
Reference
-
1. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011; 365:2205–19.
Article2. Pincus T, Gibson KA, Block JA. Premature mortality: a neglected outcome in rheumatic diseases? Arthritis Care Res (Hoboken). 2015; 67:1043–6.
Article3. Smolen JS, Landewé R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017; 76:960–77.4. Katchamart W, Trudeau J, Phumethum V, Bombardier C. Efficacy and toxicity of methotrexate (MTX) monotherapy versus MTX combination therapy with non-biological disease-modifying antirheumatic drugs in rheumatoid arthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2009; 68:1105–12.
Article5. Salliot C, van der Heijde D. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann Rheum Dis. 2009; 68:1100–4.
Article6. Kvalvik AG, Lefsaker L, Dyvik S, Brun JG. Anti-tumor necrosis factor-alpha therapy in the ordinary clinical setting: three-year effectiveness in patients with rheumatoid arthritis. Joint Bone Spine. 2007; 74:606–11.
Article7. Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003; 48:35–45.8. Gizinski AM, Fox DA. T cell subsets and their role in the pathogenesis of rheumatic disease. Curr Opin Rheumatol. 2014; 26:204–10.
Article9. Scott LJ, McKeage K, Keam SJ, Plosker GL. Tacrolimus: a further update of its use in the management of organ transplantation. Drugs. 2003; 63:1247–97.10. Ogawa T, Tokuda M, Tomizawa K, Matsui H, Itano T, Konishi R, et al. Osteoblastic differentiation is enhanced by rapamycin in rat osteoblast-like osteosarcoma (ROS 17/2.8) cells. Biochem Biophys Res Commun. 1998; 249:226–30.
Article11. Spolidorio LC, Nassar PO, Nassar CA, Spolidorio DM, Muscará MN. Conversion of immunosuppressive monotherapy from cyclosporin A to tacrolimus reverses bone loss in rats. Calcif Tissue Int. 2007; 81:114–23.
Article12. Lee WS, Lee SI, Lee MS, Kim SI, Lee SS, Yoo WH. Efficacy and safety of low-dose tacrolimus for active rheumatoid arthritis with an inadequate response to methotrexate. Korean J Intern Med. 2016; 31:779–87.
Article13. Kawai S, Takeuchi T, Yamamoto K, Tanaka Y, Miyasaka N. Efficacy and safety of additional use of tacrolimus in patients with early rheumatoid arthritis with inadequate response to DMARDs − a multicenter, double-blind, paral-lel-group trial. Mod Rheumatol. 2011; 21:458–68.14. Kawai S, Yamamoto K. Safety of tacrolimus, an immunosuppressive agent, in the treatment of rheumatoid arthritis in elderly patients. Rheumatology (Oxford). 2006; 45:441–4.
Article15. Hochberg MC, Chang RW, Dwosh I, Lindsey S, Pincus T, Wolfe F. The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheum. 1992; 35:498–502.
Article16. van der Heijde DM, van't Hof MA, van Riel PL, Theunisse LA, Lubberts EW, van Leeuwen MA, et al. Judging disease activity in clinical practice in rheumatoid arthritis: first step in the development of a disease activity score. Ann Rheum Dis. 1990; 49:916–20.
Article17. Kawai S, Hashimoto H, Kondo H, Murayama T, Kiuchi T, Abe T. Comparison of tacrolimus and mizoribine in a randomized, double-blind controlled study in patients with rheumatoid arthritis. J Rheumatol. 2006; 33:2153–61.18. Furst DE, Saag K, Fleischmann MR, Sherrer Y, Block JA, Schnitzer T, et al. Efficacy of tacrolimus in rheumatoid arthritis patients who have been treated unsuccessfully with methotrexate: a six-month, double-blind, randomized, doseranging study. Arthritis Rheum. 2002; 46:2020–8.
Article19. Kondo H, Abe T, Hashimoto H, Uchida S, Irimajiri S, Hara M, et al. Efficacy and safety of tacrolimus (FK506) in treatment of rheumatoid arthritis: a randomized, double blind, placebo controlled dose-finding study. J Rheumatol. 2004; 31:243–51.20. Kremer JM, Habros JS, Kolba KS, Kaine JL, Borton MA, Mengle-Gaw LJ, et al. Tacrolimus in rheumatoid arthritis patients receiving concomitant methotrexate: a six-month, open-label study. Arthritis Rheum. 2003; 48:2763–8.21. Yocum DE, Furst DE, Kaine JL, Baldassare AR, Stevenson JT, Borton MA, et al. Efficacy and safety of tacrolimus in patients with rheumatoid arthritis: a double-blind trial. Arthritis Rheum. 2003; 48:3328–37.
Article22. Takeuchi T, Ishida K, Shiraki K, Yoshiyasu T. Safety and effectiveness of tacrolimus add-on therapy for rheumatoid arthritis patients without an adequate response to biological disease-modifying anti-rheumatic drugs (DMARDs): postmarketing surveillance in Japan. Mod Rheumatol. 2018; 28:48–57.
Article23. Miyazaki M, Fujikawa Y, Takita C, Tsumura H. Tacrolimus and cyclosporine A inhibit human osteoclast formation via targeting the calcineurindependent NFAT pathway and an activation pathway for c-Jun or MITF in rheumatoid arthritis. Clin Rheumatol. 2007; 26:231–9.
Article24. Bae SC, Cook EF, Kim SY. Psychometric evaluation of a Korean Health Assessment Questionnaire for clinical research. J Rheumatol. 1998; 25:1975–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cementless Total Knee Arthroplasty and Effects of Disease Modifying Anti-Rheumatic Drugs in Rheumatoid Arthritis
- Efficacy and safety of low-dose tacrolimus for active rheumatoid arthritis with an inadequate response to methotrexate
- Add-on Clarithromycin and Tacrolimus Treatment for Rheumatoid Arthritis
- The efficacy and safety of etanercept in patients with active rheumatoid arthritis receiving methotrexate
- Recent trends and guidelines for the management of rheumatoid arthritis