J Breast Cancer.
2018 Mar;21(1):51-61. 10.4048/jbc.2018.21.1.51.
Clinicopathologic and Prognostic Significance of the Zinc Finger of the Cerebellum Family in Invasive Breast Cancer
- Affiliations
-
- 1Department of General Surgery, Kunshan First People's Hospital Affiliated to Jiangsu University, Kunshan, China. dinghouzhong@163.com
- 2Department of Pharmacy, Kunshan Hospital of Traditional Chinese Medicine, Kunshan, China.
- 3Department of Pathology, Kunshan First People's Hospital Affiliated to Jiangsu University, Kunshan, China.
- 4Department of General Surgery, Luan First People's Hospital, Luan, China.
- 5Department of Urinary Surgery, Kunshan Hospital of Traditional Chinese Medicine, Kunshan, China.
- 6Department of Gynecology, Kunshan First People's Hospital Affiliated to Jiangsu University, Kunshan, China.
- KMID: 2441868
- DOI: http://doi.org/10.4048/jbc.2018.21.1.51
Abstract
- PURPOSE
Five members of the zinc finger of the cerebellum (ZIC) family"”ZIC1, ZIC2, ZIC3, ZIC4, and ZIC5"”have been shown to be involved in various carcinomas. Here, we aimed to explore the clinicopathologic and prognostic roles of ZIC family members in invasive breast cancer patients using immunohistochemical analysis, western blotting analysis, and real-time quantitative polymerase chain reaction (RT-qPCR).
METHODS
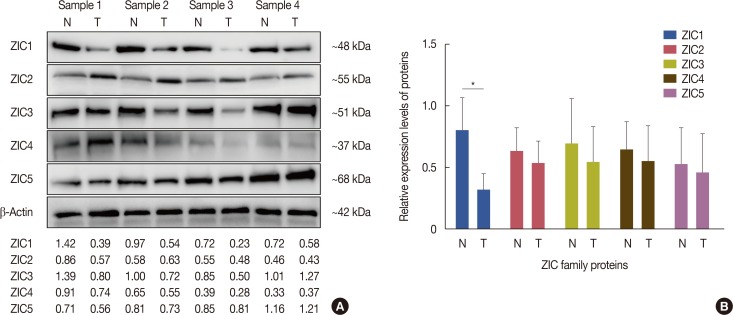
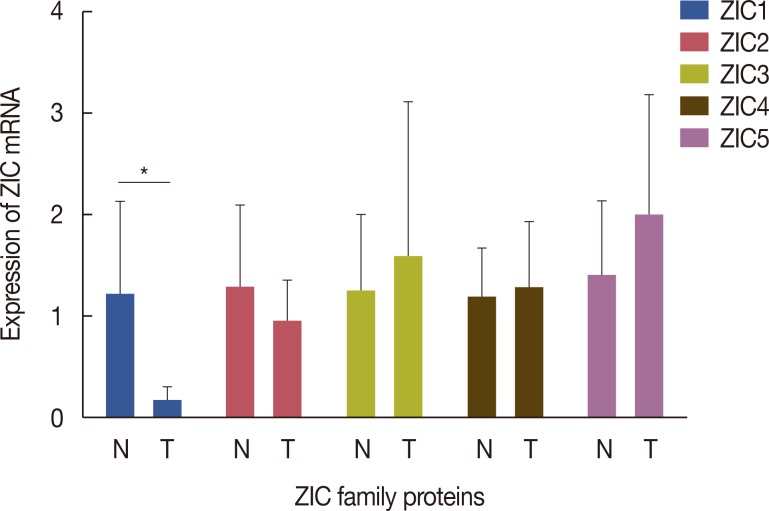
A total of 241 female invasive breast cancer patients who underwent radical mastectomy between 2009 and 2011 were enrolled. ZIC proteins in 241 pairs of breast tumors and corresponding normal tissues were investigated using immunohistochemistry and the clinicopathologic roles of proteins were analyzed using Pearson's chi-square test. Kaplan-Meier curves and Cox regression analysis were also used to analyze the prognostic value of the ZIC proteins. In addition, 12 pairs of fresh-frozen breast tumors and matched normal tissues were used in the western blotting analysis and RT-qPCR.
RESULTS
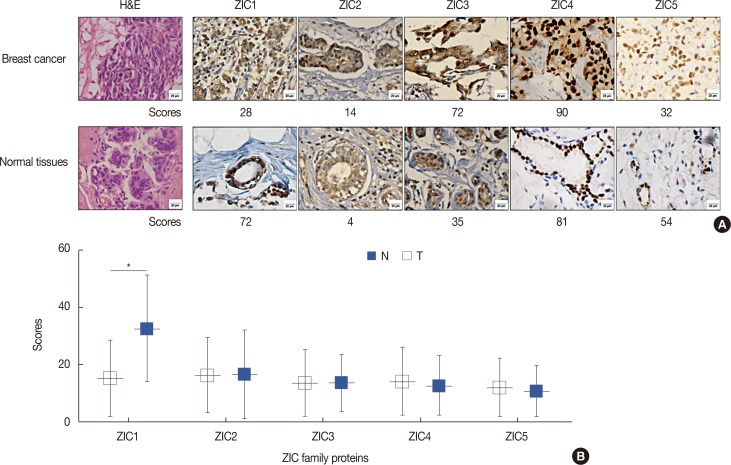
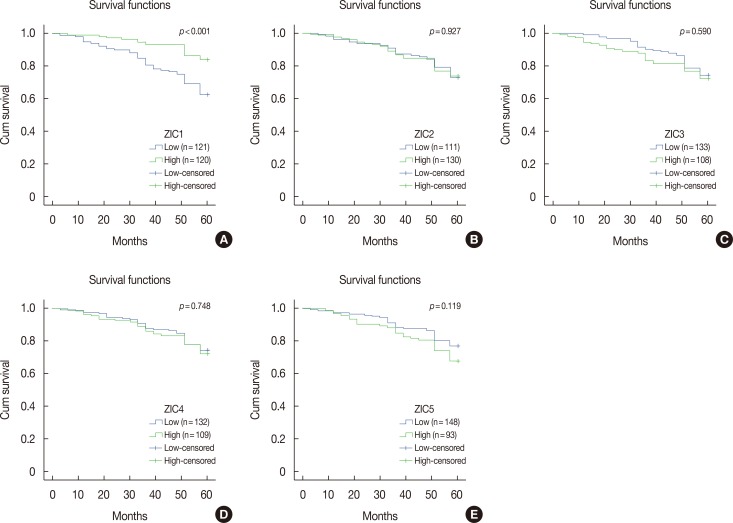
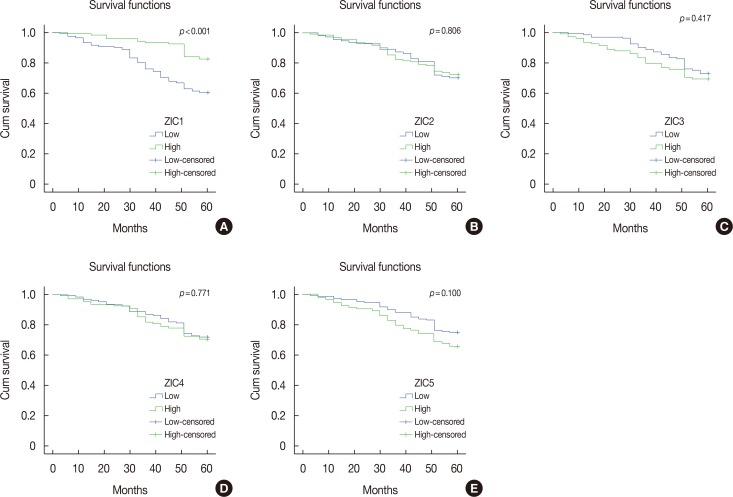
Only ZIC1 expression in normal tissues was obviously higher than that in tumors (p < 0.001). On multivariate analysis, ZIC1 expression (in overall survival analysis: hazard ratio [HR], 0.405, 95% confidence interval [CI], 0.233-0.702, p=0.001; in disease-free survival analysis: HR, 0.395, 95% CI, 0.234-0.669, p=0.001) was identified as a prognostic indicator of invasive breast cancer.
CONCLUSION
ZIC1, but not the other proteins, was obviously decreased in breast tumors and associated with clinicopathologic factors. Thus, ZIC1 might be a novel indicator to predict the overall and disease-free survival of invasive breast cancer patients.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
High Survivin and Low Zinc Finger of the Cerebellum 1 Expression Indicates Poor Prognosis in Triple-negative Breast Carcinoma
Chun-Tao Shi, Jun Ma, Qi-Feng Shi, Ye Zhang, Hao-Nan Wang
J Breast Cancer. 2019;22(2):248-259. doi: 10.4048/jbc.2019.22.e20.
Reference
-
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015; 65:5–29. PMID: 25559415.
Article2. Ali RG, Bellchambers HM, Arkell RM. Zinc fingers of the cerebellum (Zic): transcription factors and co-factors. Int J Biochem Cell Biol. 2012; 44:2065–2068. PMID: 22964024.
Article3. Aruga J, Nagai T, Tokuyama T, Hayashizaki Y, Okazaki Y, Chapman VM, et al. The mouse zic gene family: homologues of the Drosophila pair-rule gene odd-paired. J Biol Chem. 1996; 271:1043–1047. PMID: 8557628.4. Gan L, Chen S, Zhong J, Wang X, Lam EK, Liu X, et al. ZIC1 is down-regulated through promoter hypermethylation, and functions as a tumor suppressor gene in colorectal cancer. PLoS One. 2011; 6:e16916. PMID: 21347233.
Article5. Zhong J, Chen S, Xue M, Du Q, Cai J, Jin H, et al. ZIC1 modulates cell-cycle distributions and cell migration through regulation of sonic hedgehog, PI(3)K and MAPK signaling pathways in gastric cancer. BMC Cancer. 2012; 12:290. PMID: 22799764.
Article6. Qiang W, Zhao Y, Yang Q, Liu W, Guan H, Lv S, et al. ZIC1 is a putative tumor suppressor in thyroid cancer by modulating major signaling pathways and transcription factor FOXO3a. J Clin Endocrinol Metab. 2014; 99:E1163–E1172. PMID: 24684457.
Article7. Wang YY, Jiang JX, Ma H, Han J, Sun ZY, Liu ZM, et al. Role of ZIC1 methylation in hepatocellular carcinoma and its clinical significance. Tumour Biol. 2014; 35:7429–7433. PMID: 24782033.
Article8. Ma G, Dai W, Sang A, Yang X, Li Q. Roles of ZIC family genes in human gastric cancer. Int J Mol Med. 2016; 38:259–266. PMID: 27177248.
Article9. Beukers W, Kandimalla R, Masius RG, Vermeij M, Kranse R, van Leenders GJ, et al. Stratification based on methylation of TBX2 and TBX3 into three molecular grades predicts progression in patients with pTa-bladder cancer. Mod Pathol. 2015; 28:515–522. PMID: 25394776.
Article10. Kandimalla R, van Tilborg AA, Kompier LC, Stumpel DJ, Stam RW, Bangma CH, et al. Genome-wide analysis of CpG island methylation in bladder cancer identified TBX2, TBX3, GATA2, and ZIC4 as pTa-specific prognostic markers. Eur Urol. 2012; 61:1245–1256. PMID: 22284968.
Article11. Vural B, Chen LC, Saip P, Chen YT, Ustuner Z, Gonen M, et al. Frequency of SOX Group B (SOX1, 2, 3) and ZIC2 antibodies in Turkish patients with small cell lung carcinoma and their correlation with clinical parameters. Cancer. 2005; 103:2575–2583. PMID: 15880380.
Article12. Yang B, Jia L, Guo Q, Ren H, Hu D, Zhou X, et al. MiR-564 functions as a tumor suppressor in human lung cancer by targeting ZIC3. Biochem Biophys Res Commun. 2015; 467:690–696. PMID: 26498524.
Article13. Sun Q, Shi R, Wang X, Li D, Wu H, Ren B. Overexpression of ZIC5 promotes proliferation in non-small cell lung cancer. Biochem Biophys Res Commun. 2016; 479:502–509. PMID: 27663664.
Article14. Inaguma S, Ito H, Riku M, Ikeda H, Kasai K. Addiction of pancreatic cancer cells to zinc-finger transcription factor ZIC2. Oncotarget. 2015; 6:28257–28268. PMID: 26318045.
Article15. Nakakido M, Tamura K, Chung S, Ueda K, Fujii R, Kiyotani K, et al. Phosphatidylinositol glycan anchor biosynthesis, class X containing complex promotes cancer cell proliferation through suppression of EHD2 and ZIC1, putative tumor suppressors. Int J Oncol. 2016; 49:868–876. PMID: 27572108.
Article16. Pavlova TV, Kashuba VI, Muravenko OV, Yenamandra SP, Ivanova TA, Zabarovskaia VI, et al. Technology of analysis of epigenetic and structural changes of epithelial tumors genome with NotI-microarrays by the example of human chromosome. Mol Biol (Mosk). 2009; 43:339–347. PMID: 19425501.17. Belev B, Alerić I, Vrbanec D, Petrovecki M, Unusic J, Jakić-Razumović J. Nm23 gene product expression in invasive breast cancer: immunohistochemical analysis and clinicopathological correlation. Acta Oncol. 2002; 41:355–361. PMID: 12234027.18. Patel DD, Bhatavdekar JM, Chikhlikar PR, Ghosh N, Suthar TP, Shah NG, et al. Node negative breast carcinoma: hyperprolactinemia and/or overexpression of p53 as an independent predictor of poor prognosis compared to newer and established prognosticators. J Surg Oncol. 1996; 62:86–92. PMID: 8649046.
Article19. Idirisinghe PK, Thike AA, Cheok PY, Tse GM, Lui PC, Fook-Chong S, et al. Hormone receptor and c-ERBB2 status in distant metastatic and locally recurrent breast cancer. Pathologic correlations and clinical significance. Am J Clin Pathol. 2010; 133:416–429. PMID: 20154280.20. Lyu Y, Nakano K, Davis RR, Tepper CG, Campbell M, Izumiya Y. ZIC2 is essential for maintenance of latency and is a target of an immediate-early protein during KSHV lytic reactivation. J Virol. 2017; 91:e00980–e00917.
Article21. Degreef I, De Smet L, Sciot R, Cassiman JJ, Tejpar S. Immunohistochemical evidence for Zic1 coexpression with beta-catenin in the myofibroblast of Dupuytren disease. Scand J Plast Reconstr Surg Hand Surg. 2009; 43:36–40. PMID: 19153880.22. Gu X, Liu Q, Yang N, Shen JF, Zhang XG, Cao F, et al. Clinicopathological significance of increased ZIC1 expression in human endometrial cancer. J Huazhong Univ Sci Technolog Med Sci. 2015; 35:898–903. PMID: 26670443.
Article23. Brill E, Gobble R, Angeles C, Lagos-Quintana M, Crago A, Laxa B, et al. ZIC1 overexpression is oncogenic in liposarcoma. Cancer Res. 2010; 70:6891–6901. PMID: 20713527.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- High Survivin and Low Zinc Finger of the Cerebellum 1 Expression Indicates Poor Prognosis in Triple-negative Breast Carcinoma
- Prognostic Value of TZAP Expression in Various Cancers: TCGA Data Analysis
- Clinicopathological Significance of SMAD4 Expression in Breast Cancer
- Clinicopathologic Significance of p53 and c-erbB-2 Protein Expression in Breast Carcinoma
- Significance of Expression of bcl-2, p53 and cyclin D1 and Their Correlation with Clinicopathologic Prognostic Factors and Survival Rate in 128 Cases of Invasive Breast Carcinoma