J Breast Cancer.
2018 Mar;21(1):21-27. 10.4048/jbc.2018.21.1.21.
Berberine Suppresses Fibronectin Expression through Inhibition of c-Jun Phosphorylation in Breast Cancer Cells
- Affiliations
-
- 1Department of Health Sciences and Technology, Samsung Advanced Institute for Health Sciences & Technology, Sungkyunkwan University, Seoul, Korea.
- 2Breast Cancer Center, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. sangmin3005.kim@samsung.com
- 3Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2441864
- DOI: http://doi.org/10.4048/jbc.2018.21.1.21
Abstract
- PURPOSE
The exact mechanism regulating fibronectin (FN) expression in breast cancer cells has not been fully elucidated. In this study, we investigated the pharmacological mechanism of berberine (BBR) with respect to FN expression in triple-negative breast cancer (TNBC) cells.
METHODS
The clinical significance of FN mRNA expression was analyzed using the Kaplan-Meier plotter database (http://kmplot.com/breast). FN mRNA and protein expression levels were analyzed by real-time polymerase chain reaction and western blotting, respectively.
RESULTS
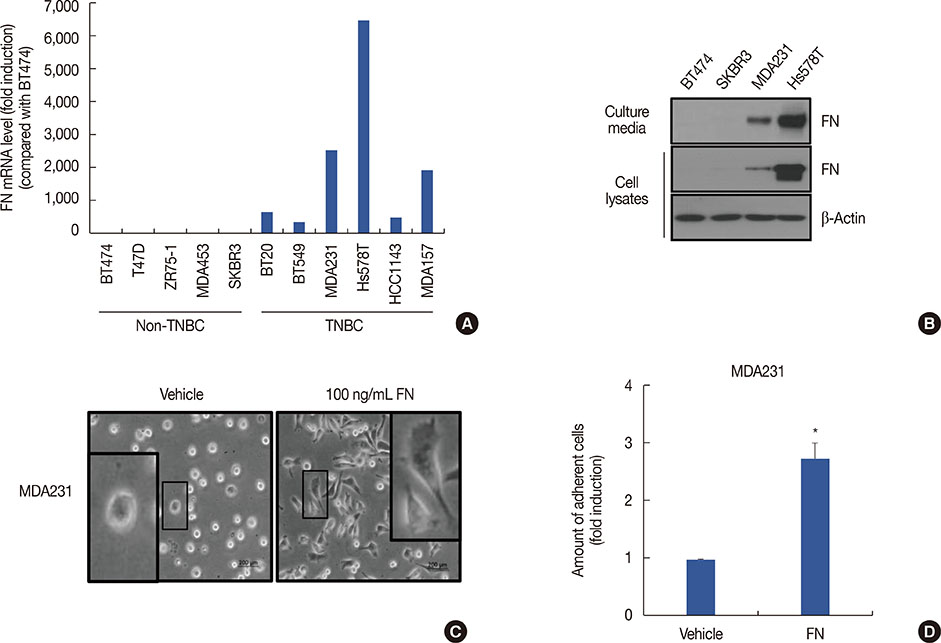
Using publicly available clinical data, we observed that high FN expression was associated with poor prognosis in patients with breast cancer. FN mRNA and protein expression was increased in TNBC cells compared with non-TNBC cells. As expected, recombinant human FN significantly induced cell spreading and adhesion in MDA-MB231 TNBC cells. We also investigated the regulatory mechanism underlying FN expression. Basal levels of FN mRNA and protein expression were downregulated by a specific activator protein-1 (AP-1) inhibitor, SR11302. Interestingly, FN expression in TNBC cells was dose-dependently decreased by BBR treatment. The level of c-Jun phosphorylation was also decreased by BBR treatment.
CONCLUSION
Our findings demonstrate that FN expression is regulated via an AP-1-dependent mechanism, and that BBR suppresses FN expression in TNBC cells through inhibition of AP-1 activity.
MeSH Terms
Figure
Reference
-
1. Ikram M. A review on the chemical and pharmacological aspects of genus Berberis. Planta Med. 1975; 28:353–358.
Article2. Liu Z, Liu Q, Xu B, Wu J, Guo C, Zhu F, et al. Berberine induces p53-dependent cell cycle arrest and apoptosis of human osteosarcoma cells by inflicting DNA damage. Mutat Res. 2009; 662:75–83.
Article3. Kim JB, Lee KM, Ko E, Han W, Lee JE, Shin I, et al. Berberine inhibits growth of the breast cancer cell lines MCF-7 and MDA-MB-231. Planta Med. 2008; 74:39–42.
Article4. Kim S, Han J, Lee SK, Choi MY, Kim J, Lee J, et al. Berberine suppresses the TPA-induced MMP-1 and MMP-9 expressions through the inhibition of PKC-alpha in breast cancer cells. J Surg Res. 2012; 176:e21–e29.5. Li X, Zhao SJ, Shi HL, Qiu SP, Xie JQ, Wu H, et al. Berberine hydrochloride IL-8 dependently inhibits invasion and IL-8-independently promotes cell apoptosis in MDA-MB-231 cells. Oncol Rep. 2014; 32:2777–2788.
Article6. Ho YT, Yang JS, Li TC, Lin JJ, Lin JG, Lai KC, et al. Berberine suppresses in vitro migration and invasion of human SCC-4 tongue squamous cancer cells through the inhibitions of FAK, IKK, NF-kappaB, u-PA and MMP-2 and -9. Cancer Lett. 2009; 279:155–162.
Article7. Jabbarzadeh Kaboli P, Rahmat A, Ismail P, Ling KH. Targets and mechanisms of berberine, a natural drug with potential to treat cancer with special focus on breast cancer. Eur J Pharmacol. 2014; 740:584–595.
Article8. Liang KW, Ting CT, Yin SC, Chen YT, Lin SJ, Liao JK, et al. Berberine suppresses MEK/ERK-dependent Egr-1 signaling pathway and inhibits vascular smooth muscle cell regrowth after in vitro mechanical injury. Biochem Pharmacol. 2006; 71:806–817.
Article9. Kim S, Oh SJ, Lee J, Han J, Jeon M, Jung T, et al. Berberine suppresses TPA-induced fibronectin expression through the inhibition of VEGF secretion in breast cancer cells. Cell Physiol Biochem. 2013; 32:1541–1550.
Article10. Hsiong SX, Huebsch N, Fischbach C, Kong HJ, Mooney DJ. Integrin-adhesion ligand bond formation of preosteoblasts and stem cells in three-dimensional RGD presenting matrices. Biomacromolecules. 2008; 9:1843–1851.
Article11. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008; 454:436–444.
Article12. Christensen L. The distribution of fibronectin, laminin and tetranectin in human breast cancer with special attention to the extracellular matrix. APMIS Suppl. 1992; 26:1–39.13. Main AL, Harvey TS, Baron M, Boyd J, Campbell ID. The three-dimensional structure of the tenth type III module of fibronectin: an insight into RGD-mediated interactions. Cell. 1992; 71:671–678.
Article14. Nagai T, Yamakawa N, Aota S, Yamada SS, Akiyama SK, Olden K, et al. Monoclonal antibody characterization of two distant sites required for function of the central cell-binding domain of fibronectin in cell adhesion, cell migration, and matrix assembly. J Cell Biol. 1991; 114:1295–1305.
Article15. Jeon M, Lee J, Nam SJ, Shin I, Lee JE, Kim S. Induction of fibronectin by HER2 overexpression triggers adhesion and invasion of breast cancer cells. Exp Cell Res. 2015; 333:116–126.
Article16. Györffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010; 123:725–731.
Article17. Helleman J, Jansen MP, Ruigrok-Ritstier K, van Staveren IL, Look MP, Meijer-van Gelder ME, et al. Association of an extracellular matrix gene cluster with breast cancer prognosis and endocrine therapy response. Clin Cancer Res. 2008; 14:5555–5564.
Article18. Nam JM, Onodera Y, Bissell MJ, Park CC. Breast cancer cells in three-dimensional culture display an enhanced radioresponse after coordinate targeting of integrin alpha5beta1 and fibronectin. Cancer Res. 2010; 70:5238–5248.
Article19. Choate JJ, Mosher DF. Fibronectin concentration in plasma of patients with breast cancer, colon cancer, and acute leukemia. Cancer. 1983; 51:1142–1147.
Article20. Zerlauth G, Wolf G. Plasma fibronectin as a marker for cancer and other diseases. Am J Med. 1984; 77:685–689.
Article21. Akiyama SK, Olden K, Yamada KM. Fibronectin and integrins in invasion and metastasis. Cancer Metastasis Rev. 1995; 14:173–189.
Article22. Bradshaw MJ, Smith ML. Multiscale relationships between fibronectin structure and functional properties. Acta Biomater. 2014; 10:1524–1531.
Article23. Mierke CT, Frey B, Fellner M, Herrmann M, Fabry B. Integrin alpha-5beta1 facilitates cancer cell invasion through enhanced contractile forces. J Cell Sci. 2011; 124:369–383.
Article24. Meng XN, Jin Y, Yu Y, Bai J, Liu GY, Zhu J, et al. Characterisation of fibronectin-mediated FAK signalling pathways in lung cancer cell migration and invasion. Br J Cancer. 2009; 101:327–334.
Article25. Lou X, Han X, Jin C, Tian W, Yu W, Ding D, et al. SOX2 targets fibronectin 1 to promote cell migration and invasion in ovarian cancer: new molecular leads for therapeutic intervention. OMICS. 2013; 17:510–518.
Article26. Tamura K, Nyui N, Tamura N, Fujita T, Kihara M, Toya Y, et al. Mechanism of angiotensin II-mediated regulation of fibronectin gene in rat vascular smooth muscle cells. J Biol Chem. 1998; 273:26487–26496.
Article27. Kumazaki T, Mitsui Y. Alterations in transcription factor-binding activities to fibronectin promoter during aging of vascular endothelial cells. Mech Ageing Dev. 1996; 88:111–124.
Article28. Beier UH, Holtmeier C, Weise JB, Görögh T. Fibronectin suppression in head and neck cancers, inflammatory tissues and the molecular mechanisms potentially involved. Int J Oncol. 2007; 30:621–629.
Article29. Mantena SK, Sharma SD, Katiyar SK. Berberine inhibits growth, induces G1 arrest and apoptosis in human epidermoid carcinoma A431 cells by regulating Cdki-Cdk-cyclin cascade, disruption of mitochondrial membrane potential and cleavage of caspase 3 and PARP. Carcinogenesis. 2006; 27:2018–2027.
Article30. Hsu WH, Hsieh YS, Kuo HC, Teng CY, Huang HI, Wang CJ, et al. Berberine induces apoptosis in SW620 human colonic carcinoma cells through generation of reactive oxygen species and activation of JNK/p38 MAPK and FasL. Arch Toxicol. 2007; 81:719–728.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Berberine Suppresses Interleukin-1beta-Induced MUC5AC Gene Expression in Human Airway Epithelial Cells
- The inhibition of inflammatory molecule expression on 3T3-L1 adipocytes by berberine is not mediated by leptin signaling
- Activating transcription factor-3 induction is involved in the anti-inflammatory action of berberine in RAW264.7 murine macrophages
- Alfa - difluoromethylornithine Reduced Protein Phosphorylation in MCF-7 Human Breast Cancer Cells
- Berberine Inhibited the Growth of Thyroid Cancer Cell Lines 8505C and TPC1