Allergy Asthma Immunol Res.
2018 Nov;10(6):614-627. 10.4168/aair.2018.10.6.614.
Lung Function Trajectory Types in Never-Smoking Adults With Asthma: Clinical Features and Inflammatory Patterns
- Affiliations
-
- 1Division of Pulmonology, Allergy and Critical Care, Department of Internal Medicine, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea.
- 2Genome Research Center for Allergy and Respiratory Diseases, Bucheon, Korea.
- 3Department of Interdisciplinary Program in Biomedical Science Major, Soonchunhyang Graduate School, Asan, Korea.
- 4Division of Allergy and Respiratory Medicine, Department of Internal Medicine, Soonchunhyang University Bucheon Hospital, Bucheon, Korea. js1221@schmc.ac.kr
- KMID: 2441812
- DOI: http://doi.org/10.4168/aair.2018.10.6.614
Abstract
- PURPOSE
Asthma is a heterogeneous disease that responds to medications to varying degrees. Cluster analyses have identified several phenotypes and variables related to fixed airway obstruction; however, few longitudinal studies of lung function have been performed on adult asthmatics. We investigated clinical, demographic, and inflammatory factors related to persistent airflow limitation based on lung function trajectories over 1 year.
METHODS
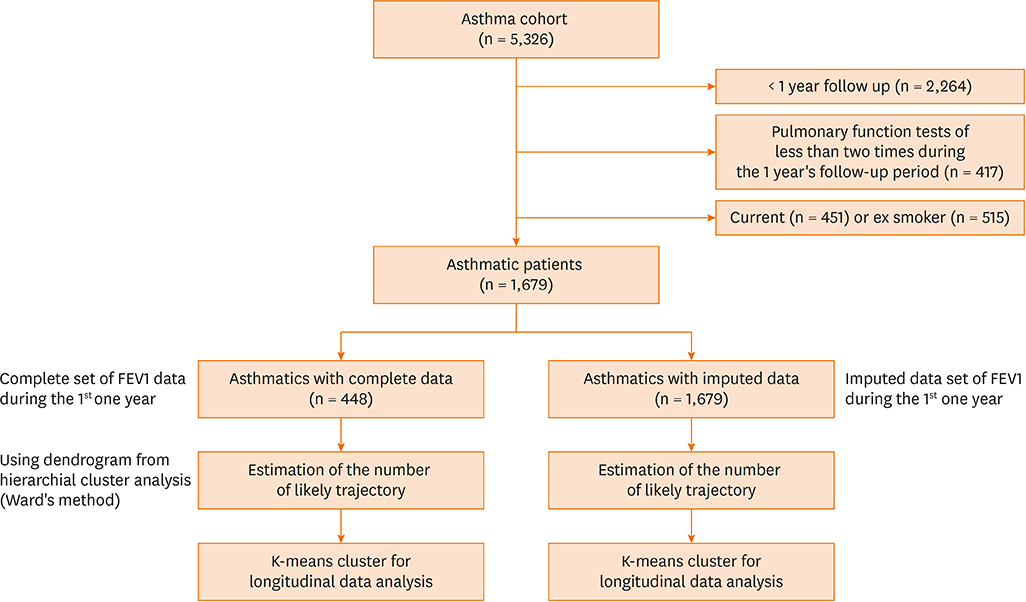
Serial post-bronchodilator forced expiratory volume (FEV) 1% values were obtained from 1,679 asthmatics who were followed up every 3 months for 1 year. First, a hierarchical cluster analysis was performed using Ward's method to generate a dendrogram for the optimum number of clusters using the complete post-FEV1 sets from 448 subjects. Then, a trajectory cluster analysis of serial post-FEV1 sets was performed using the k-means clustering for the longitudinal data trajectory method. Next, trajectory clustering for the serial post-FEV1 sets of a total of 1,679 asthmatics was performed after imputation of missing post-FEV1 values using regression methods.
RESULTS
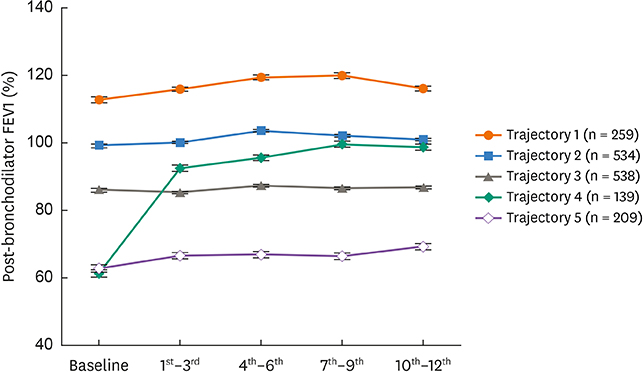
Trajectories 1 and 2 were associated with normal lung function during the study period, and trajectory 3 was associated with a reversal to normal of the moderately decreased baseline FEV1 within 3 months. Trajectories 4 and 5 were associated with severe asthma with a marked reduction in baseline FEV1. However, the FEV1 associated with trajectory 4 was increased at 3 months, whereas the FEV1 associated with trajectory 5 was persistently disturbed over 1 year. Compared with trajectory 4, trajectory 5 was associated with older asthmatics with less atopy, a lower immunoglobulin E (IgE) level, sputum neutrophilia and higher dosages of oral steroids. In contrast, trajectory 4 was associated with higher sputum and blood eosinophil counts and more frequent exacerbations.
CONCLUSIONS
Trajectory clustering analysis of FEV1 identified 5 distinct types, representing well-preserved to severely decreased FEV1. Persistent airflow obstruction may be related to non-atopy, a low IgE level, and older age accompanied by neutrophilic inflammation and low baseline FEV1 levels.
MeSH Terms
Figure
Cited by 1 articles
-
Is a Longitudinal Trajectory Helpful in Identifying Phenotypes in Asthma?
Tae-Bum Kim
Allergy Asthma Immunol Res. 2018;10(6):571-574. doi: 10.4168/aair.2018.10.6.571.
Reference
-
1. Reddel HK, Bateman ED, Becker A, Boulet LP, Cruz AA, Drazen JM, et al. A summary of the new GINA strategy: a roadmap to asthma control. Eur Respir J. 2015; 46:622–639.
Article2. Fajt ML, Wenzel SE. Development of new therapies for severe asthma. Allergy Asthma Immunol Res. 2017; 9:3–14.
Article3. Sears MR. Lung function decline in asthma. Eur Respir J. 2007; 30:411–413.
Article4. Newby C, Agbetile J, Hargadon B, Monteiro W, Green R, Pavord I, et al. Lung function decline and variable airway inflammatory pattern: longitudinal analysis of severe asthma. J Allergy Clin Immunol. 2014; 134:287–294.
Article5. Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010; 181:315–323.
Article6. Shaw DE, Berry MA, Hargadon B, McKenna S, Shelley MJ, Green RH, et al. Association between neutrophilic airway inflammation and airflow limitation in adults with asthma. Chest. 2007; 132:1871–1875.
Article7. Amelink M, de Groot JC, de Nijs SB, Lutter R, Zwinderman AH, Sterk PJ, et al. Severe adult-onset asthma: a distinct phenotype. J Allergy Clin Immunol. 2013; 132:336–341.
Article8. Chung KF. Neutrophilic asthma: a distinct target for treatment? Lancet Respir Med. 2016; 4:765–767.
Article9. Chang HS, Lee TH, Jun JA, Baek AR, Park JS, Koo SM, et al. Neutrophilic inflammation in asthma: mechanisms and therapeutic considerations. Expert Rev Respir Med. 2017; 11:29–40.
Article10. Newby C, Heaney LG, Menzies-Gow A, Niven RM, Mansur A, Bucknall C, et al. Statistical cluster analysis of the British Thoracic Society Severe refractory Asthma Registry: clinical outcomes and phenotype stability. PLoS One. 2014; 9:e102987.
Article11. Loza MJ, Djukanovic R, Chung KF, Horowitz D, Ma K, Branigan P, et al. Validated and longitudinally stable asthma phenotypes based on cluster analysis of the ADEPT study. Respir Res. 2016; 17:165.
Article12. Boudier A, Curjuric I, Basagaña X, Hazgui H, Anto JM, Bousquet J, et al. Ten-year follow-up of cluster-based asthma phenotypes in adults. A pooled analysis of three cohorts. Am J Respir Crit Care Med. 2013; 188:550–560.
Article13. Kim TB, Jang AS, Kwon HS, Park JS, Chang YS, Cho SH, et al. Identification of asthma clusters in two independent Korean adult asthma cohorts. Eur Respir J. 2013; 41:1308–1314.
Article14. Park SY, Baek S, Kim S, Yoon SY, Kwon HS, Chang YS, et al. Clinical significance of asthma clusters by longitudinal analysis in Korean asthma cohort. PLoS One. 2013; 8:e83540.
Article15. Savenije OE, Granell R, Caudri D, Koppelman GH, Smit HA, Wijga A, et al. Comparison of childhood wheezing phenotypes in 2 birth cohorts: ALSPAC and PIAMA. J Allergy Clin Immunol. 2011; 127:1505–1512.e14.
Article16. Panico L, Stuart B, Bartley M, Kelly Y. Asthma trajectories in early childhood: identifying modifiable factors. PLoS One. 2014; 9:e111922.
Article17. Lødrup Carlsen KC, Mowinckel P, Hovland V, Håland G, Riiser A, Carlsen KH. Lung function trajectories from birth through puberty reflect asthma phenotypes with allergic comorbidity. J Allergy Clin Immunol. 2014; 134:917–923.e7.
Article18. Bisgaard H, Jensen SM, Bønnelykke K. Interaction between asthma and lung function growth in early life. Am J Respir Crit Care Med. 2012; 185:1183–1189.
Article19. Rasmussen F, Taylor DR, Flannery EM, Cowan JO, Greene JM, Herbison GP, et al. Risk factors for airway remodeling in asthma manifested by a low postbronchodilator FEV1/vital capacity ratio: a longitudinal population study from childhood to adulthood. Am J Respir Crit Care Med. 2002; 165:1480–1488.20. Guerra S, Sherrill DL, Kurzius-Spencer M, Venker C, Halonen M, Quan SF, et al. The course of persistent airflow limitation in subjects with and without asthma. Respir Med. 2008; 102:1473–1482.
Article21. Choi JS, Jang AS, Park JS, Park SW, Paik SH, Park JS, et al. Role of neutrophils in persistent airway obstruction due to refractory asthma. Respirology. 2012; 17:322–329.
Article22. Kim DK, Park YB, Oh YM, Jung KS, Yoo JH, Yoo KH, et al. Korean Asthma Guideline 2014: summary of major updates to the Korean Asthma Guideline 2014. Tuberc Respir Dis (Seoul). 2016; 79:111–120.
Article23. Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009; 180:59–99.24. Everitt BS. Cluster Analysis. 3rd ed. New York: John Wiley;1993.25. Genolini C, Falissard B. KmL: a package to cluster longitudinal data. Comput Methods Programs Biomed. 2011; 104:e112–e121.
Article26. Tepper RS, Wise RS, Covar R, Irvin CG, Kercsmar CM, Kraft M, et al. Asthma outcomes: pulmonary physiology. J Allergy Clin Immunol. 2012; 129:S65–87.
Article27. Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005; 26:948–968.28. Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014; 43:343–373.29. Engelkes M, Janssens HM, de Jongste JC, Sturkenboom MC, Verhamme KM. Medication adherence and the risk of severe asthma exacerbations: a systematic review. Eur Respir J. 2015; 45:396–407.
Article30. Tay TR, Hew M. Comorbid “treatable traits” in difficult asthma: current evidence and clinical evaluation. Allergy. 2017.
Article31. Fahy JV. Eosinophilic and neutrophilic inflammation in asthma: insights from clinical studies. Proc Am Thorac Soc. 2009; 6:256–259.
Article32. Kupczyk M, ten Brinke A, Sterk PJ, Bel EH, Papi A, Chanez P, et al. Frequent exacerbators--a distinct phenotype of severe asthma. Clin Exp Allergy. 2014; 44:212–221.33. van Veen IH, Ten Brinke A, Gauw SA, Sterk PJ, Rabe KF, Bel EH. Consistency of sputum eosinophilia in difficult-to-treat asthma: a 5-year follow-up study. J Allergy Clin Immunol. 2009; 124:615–617.
Article34. Chung KF. Targeting the interleukin pathway in the treatment of asthma. Lancet. 2015; 386:1086–1096.
Article35. Shaw DE, Sousa AR, Fowler SJ, Fleming LJ, Roberts G, Corfield J, et al. Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur Respir J. 2015; 46:1308–1321.36. Kim TB, Jang AS, Kwon HS, Park JS, Chang YS, Cho SH, et al. Identification of asthma clusters in two independent Korean adult asthma cohorts. Eur Respir J. 2013; 41:1308–1314.
Article37. Lee T, Lee YS, Bae YJ, Kim TB, Kim SO, Cho SH, et al. Smoking, longer disease duration and absence of rhinosinusitis are related to fixed airway obstruction in Koreans with severe asthma: findings from the COREA study. Respir Res. 2011; 12:1.
Article38. Cerveri I, Cazzoletti L, Corsico AG, Marcon A, Niniano R, Grosso A, et al. The impact of cigarette smoking on asthma: a population-based international cohort study. Int Arch Allergy Immunol. 2012; 158:175–183.
Article39. Thomson NC, Chaudhuri R, Heaney LG, Bucknall C, Niven RM, Brightling CE, et al. Clinical outcomes and inflammatory biomarkers in current smokers and exsmokers with severe asthma. J Allergy Clin Immunol. 2013; 131:1008–1016.
Article40. Gibson PG, McDonald VM. Asthma-COPD overlap 2015: now we are six. Thorax. 2015; 70:683–691.
Article41. Montnémery P, Hansson L, Lanke J, Lindholm LH, Nyberg P, Löfdahl CG, et al. Accuracy of a first diagnosis of asthma in primary health care. Fam Pract. 2002; 19:365–368.42. Lucas AE, Smeenk FW, Smeele IJ, van Schayck CP. Overtreatment with inhaled corticosteroids and diagnostic problems in primary care patients, an exploratory study. Fam Pract. 2008; 25:86–91.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The association between smoking and asthma
- Effects of Paternal Tobacco Smoking on Children's Pulmonary Function and Prevalence of Asthma and Lifetime Wheezing Episode
- The Relationship between Smoking and Pulmonary Function Test by Body Mass Index and Age: The Korean National Health and Nutrition Survey
- Pulmonary Function and Clinical Characteristics Influenced by Cigarette Smoking among Adult Asthmatics
- Trajectories of Cognitive Function and Their Associated Factors in Community-Dwelling Older Adults by Living Arrangement Using the Korean Longitudinal Study of Aging