J Korean Neurosurg Soc.
2019 Mar;62(2):183-192. 10.3340/jkns.2017.0314.
Patient-Specific Computational Fluid Dynamics in Ruptured Posterior Communicating Aneurysms Using Measured Non-Newtonian Viscosity : A Preliminary Study
- Affiliations
-
- 1Department of Bionanosystem Engineering, Chonbuk National University, Jeonju, Korea.
- 2Division of Mechanical Design Engineering, Chonbuk National University, Jeonju, Korea. 0311dhlee@jbnu.ac.kr
- 3Hemorheology Research Institute, Chonbuk National University, Jeonju, Korea.
- 4Department of Radiology, Research Institute of Clinical Medicine of Chonbuk National University-Biomedical Research Institute of Chonbuk National University Hospital, Jeonju, Korea. kwak8141@jbnu.ac.kr
- 5Department of Neurosurgery, Research Institute of Clinical Medicine of Chonbuk National University-Biomedical Research Institute of Chonbuk National University Hospital, Jeonju, Korea.
- KMID: 2441562
- DOI: http://doi.org/10.3340/jkns.2017.0314
Abstract
OBJECTIVE
The objective of this study was to analyze patient-specific blood flow in ruptured aneurysms using obtained non-Newtonian viscosity and to observe associated hemodynamic features and morphological effects.
METHODS
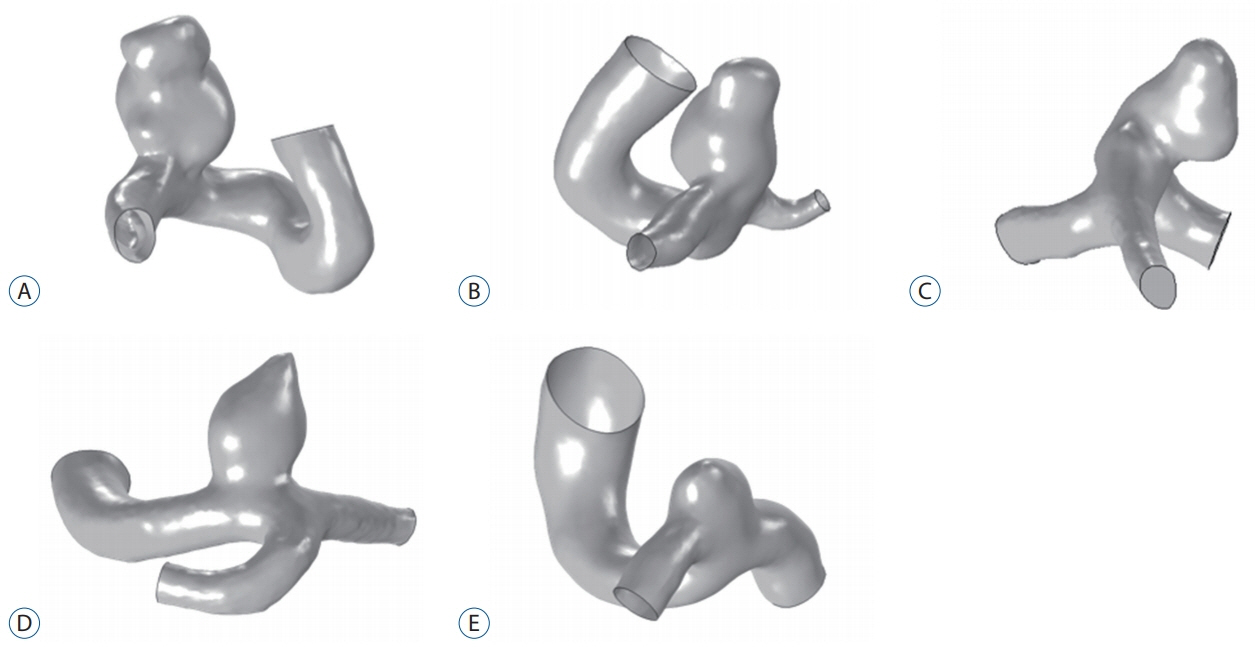
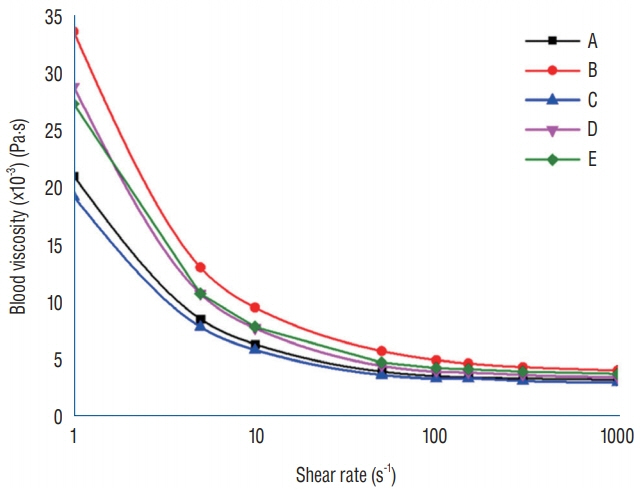
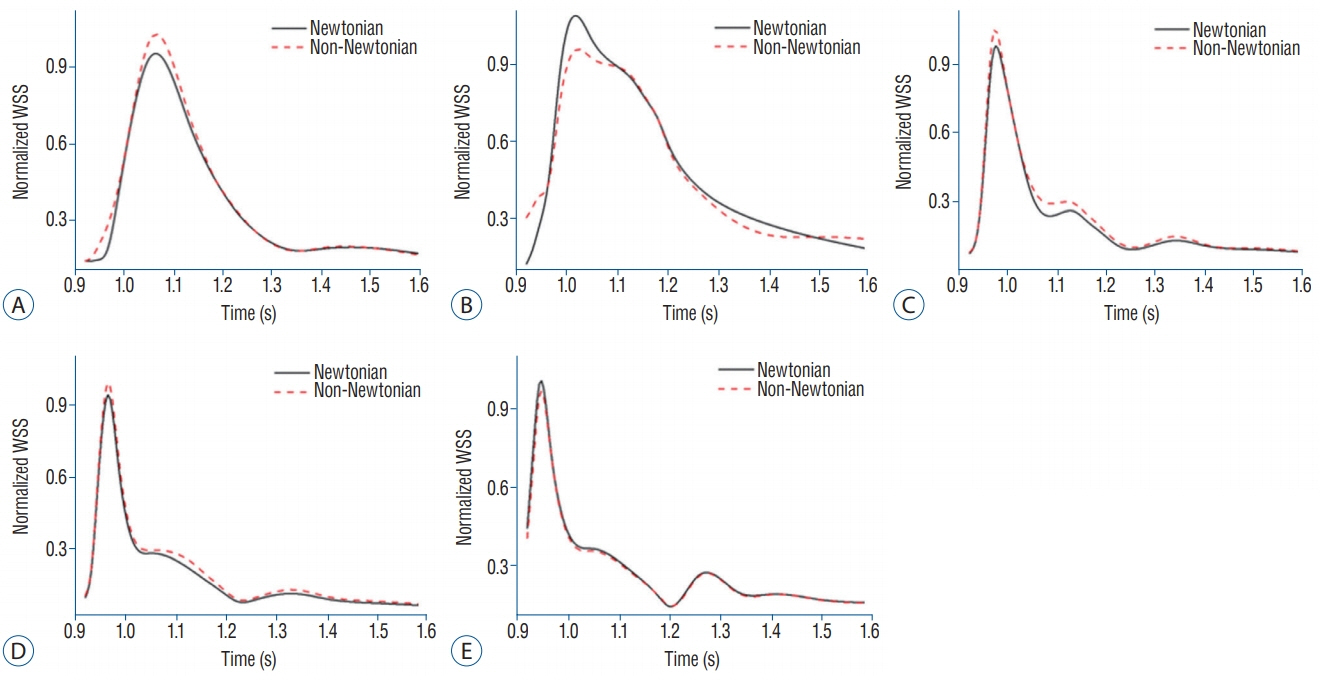
Five patients with acute subarachnoid hemorrhage caused by ruptured posterior communicating artery aneurysms were included in the study. Patients' blood samples were measured immediately after enrollment. Computational fluid dynamics (CFD) was conducted to evaluate viscosity distributions and wall shear stress (WSS) distributions using a patient-specific geometric model and shear-thinning viscosity properties.
RESULTS
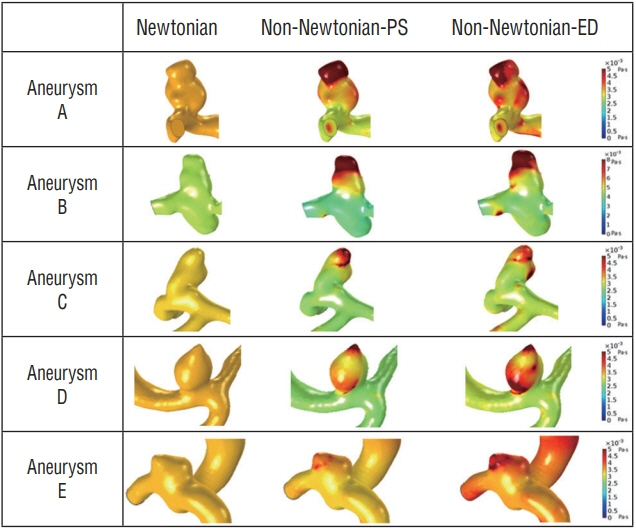
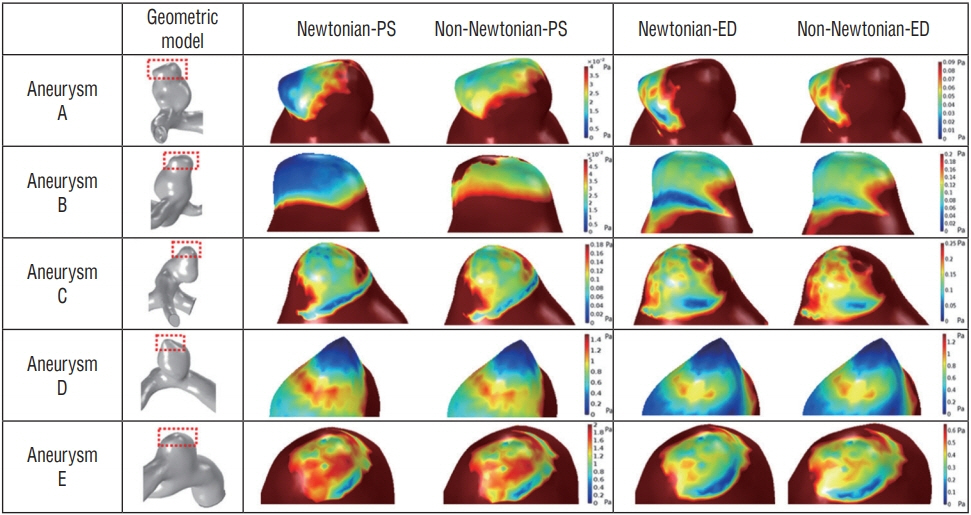
Substantial viscosity change was found at the dome of the aneurysms studied when applying non-Newtonian blood viscosity measured at peak-systole and end-diastole. The maximal WSS of the non-Newtonian model on an aneurysm at peaksystole was approximately 16% lower compared to Newtonian fluid, and most of the hemodynamic features of Newtonian flow at the aneurysms were higher, except for minimal WSS value. However, the differences between the Newtonian and non-Newtonian flow were not statistically significant. Rupture point of an aneurysm showed low WSS regardless of Newtonian or non-Newtonian CFD analyses.
CONCLUSION
By using measured non-Newtonian viscosity and geometry on patient-specific CFD analysis, morphologic differences in hemodynamic features, such as changes in whole blood viscosity and WSS, were observed. Therefore, measured non-Newtonian viscosity might be possibly useful to obtain patient-specific hemodynamic and morphologic result.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Aroesty J, Gross JF. The mathematics of pulsatile flow in small vessels. I. Casson theory. Microvasc Res. 4:1–12. 1972.
Article2. Baskurt OK, Meiselman HJ. Blood rheology and hemodynamics. Semin Thromb Hemost. 29:435–450. 2003.
Article3. Castro MA, Putman CM, Sheridan MJ, Cebral JR. Hemodynamic patterns of anterior communicating artery aneurysms: a possible association with rupture. AJNR Am J Neuroradiol. 30:297–302. 2009.
Article4. Cebral JR, Castro MA, Appanaboyina S, Putman CM, Millan D, Frangi AF. Efficient pipeline for image-based patient-specific analysis of cerebral aneurysm hemodynamics: technique and sensitivity. IEEE Trans Med Imaging. 24:457–467. 2005.
Article5. Cebral JR, Castro MA, Burgess JE, Pergolizzi RS, Sheridan MJ, Putman CM. Characterization of cerebral aneurysms for assessing risk of rupture by using patient-specific computational hemodynamics models. AJNR Am J Neuroradiol. 26:2550–2559. 2005.6. Chaturani P, Samy RP. Pulsatile flow of Casson’s fluid through stenosed arteries with applications to blood flow. Biorheology. 23:499–511. 1986.
Article7. Chien A, Castro M, Tateshima S, Sayre J, Cebral J, Viñuela F. Quantitative hemodynamic analysis of brain aneurysms at different locations. AJNR Am J Neuroradiol. 30:1507–1512. 2009.
Article8. Cho YI, Kensey KR. Effects of the non-Newtonian viscosity of blood on flows in a diseased arterial vessel. Part 1: steady flows. Biorheology. 28:241–262. 1991.
Article9. Crawford T. Some observations on the pathogenesis and natural history of intracranial aneurysms. J Neurol Neurosurg Psychiatry. 22:259–266. 1959.
Article10. Crompton MR. Mechanism of growth and rupture in cerebral berry aneurysms. Br Med J. 1:1138–1142. 1966.
Article11. Errill EW. Rheology of blood. Physiol Rev. 49:863–888. 1969.
Article12. Fukazawa K, Ishida F, Umeda Y, Miura Y, Shimosaka S, Matsushima S, et al. Using computational fluid dynamics analysis to characterize local hemodynamic features of middle cerebral artery aneurysm rupture points. World Neurosurg. 83:80–86. 2015.
Article13. Gijsen FJ, van de Vosse FN, Janssen JD. The influence of the non-Newtonian properties of blood on the flow in large arteries: steady flow in a carotid bifurcation model. J Biomech. 32:601–608. 1999.
Article14. Hilzenrat N, Arish A, Yaari A, Almog Y, Sikuler E. Blood viscosity, hemodynamics and vascular hindrance in a rat model of acute controlled bleeding and volume restitution with blood or Haemaccel. Acta Anaesthesiol Scand. 45:371–376. 2001.
Article15. Hilzenrat N, Arish A, Yaari A, Sikuler E. Acute hemodynamic changes following hemorrhage and volume restitution, using a low viscosity plasma expander, in anesthetized portal hypertensive rats. J Hepatol. 31:874–879. 1999.
Article16. Hodis S, Uthamaraj S, Lanzino G, Kallmes DF, Dragomir-Daescu D. Computational fluid dynamics simulation of an anterior communicating artery ruptured during angiography. J Neurointerv Surg. 6:e14. 2014.
Article17. Hopkins RW, Fratianne RB, Rao KV, Damewood CA. Effects of hematocrit and viscosity on continuing hemorrhage. J Trauma. 14:482–493. 1974.
Article18. Ishii R. Regional cerebral blood flow in patients with ruptured intracranial aneurysms. J Neurosurg. 50:587–594. 1979.
Article19. Jansen I, Schneiders J, Potters W, van Ooij P, van den Berg R, van Bavel E, et al. Generalized versus patient-specific inflow boundary conditions in computational fluid dynamics simulations of cerebral aneurysmal hemodynamics. AJNR Am J Neuroradiol. 35:1543–1548. 2014.
Article20. Jou LD, Lee DH, Morsi H, Mawad ME. Wall shear stress on ruptured and unruptured intracranial aneurysms at the internal carotid artery. AJNR Am J Neuroradiol. 29:1761–1767. 2008.
Article21. Jung JM, Lee DH, Kim KT, Choi MS, Cho YG, Lee HS, et al. Reference intervals for whole blood viscosity using the analytical performance-evaluated scanning capillary tube viscometer. Clin Biochem. 47:489–493. 2014.
Article22. Karmonik C, Zhang YJ, Diaz O, Klucznik R, Partovi S, Grossman RG, et al. Magnetic resonance imaging as a tool to assess reliability in simulating hemodynamics in cerebral aneurysms with a dedicated computational fluid dynamics prototype: preliminary results. Cardiovasc Diagn Ther. 4:207–212. 2014.23. Le WJ, Zhu YQ, Li MH, Yan L, Tan HQ, Xiao SM, et al. New method for retrospective study of hemodynamic changes before and after aneurysm formation in patients with ruptured or unruptured aneurysms. BMC Neurol. 13:166. 2013.
Article24. Meng H, Wang Z, Hoi Y, Gao L, Metaxa E, Swartz DD, et al. Complex hemodynamics at the apex of an arterial bifurcation induces vascular remodeling resembling cerebral aneurysm initiation. Stroke. 38:1924–1931. 2007.
Article25. Morales HG, Larrabide I, Geers AJ, Aguilar ML, Frangi AF. Newtonian and non-Newtonian blood flow in coiled cerebral aneurysms. J Biomech. 46:2158–2164. 2013.
Article26. Nicodemo L, Nicolais L, Landel RF. Shear rate dependent viscosity of suspensions in Newtonian and non-Newtonian liquids. Chem Eng Sci. 29:729–735. 1974.
Article27. Nixon AM, Gunel M, Sumpio BE. The critical role of hemodynamics in the development of cerebral vascular disease: a review. J Neurosurg. 112:1240–1253. 2010.
Article28. Ohkuma H, Manabe H, Tanaka M, Suzuki S. Impact of cerebral microcirculatory changes on cerebral blood flow during cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 31:1621–1627. 2000.
Article29. Perktold K, Peter R, Resch M. Pulsatile non-Newtonian blood flow simulation through a bifurcation with an aneurysm. Biorheology. 26:1011–1030. 1989.
Article30. Rayz VL, Boussel L, Lawton MT, Acevedo-Bolton G, Ge L, Young WL, et al. Numerical modeling of the flow in intracranial aneurysms: prediction of regions prone to thrombus formation. Ann Biomed Eng. 36:1793–1804. 2008.
Article31. Sankar D, Hemalatha K. A non-Newtonian fluid flow model for blood flow through a catheterized artery—steady flow. Appl Math Model. 31:1847–1864. 2007.
Article32. Shojima M, Oshima M, Takagi K, Torii R, Hayakawa M, Katada K, et al. Magnitude and role of wall shear stress on cerebral aneurysm: computational fluid dynamic study of 20 middle cerebral artery aneurysms. Stroke. 35:2500–2505. 2004.
Article33. Solnordal CB, Liovic P, Delaney GW, Cummins SJ, Schwarz MP, Witt PJ, editors. CFD sensitivity study for Newtonian viscosity model in cerebral aneurysms. Eleventh international conference on computational fluid dynamics in the minerals and process industries. 2015 Dec 7-9; Melbourne, Australia. Melbourne: CSIRO; 2015 Dec.34. Takao H, Murayama Y, Otsuka S, Qian Y, Mohamed A, Masuda S, et al. Hemodynamic differences between unruptured and ruptured intracranial aneurysms during observation. Stroke. 43:1436–1439. 2012.
Article35. Valen-Sendstad K, Steinman DA. Mind the gap: impact of computational fluid dynamics solution strategy on prediction of intracranial aneurysm hemodynamics and rupture status indicators. AJNR Am J Neuroradiol. 35:536–543. 2014.
Article36. Valencia A, Zarate A, Galvez M, Badilla L. Non‐Newtonian blood flow dynamics in a right internal carotid artery with a saccular aneurysm. Int J Numer Methods Fluids. 50:751–764. 2006.
Article37. Xiang J, Natarajan SK, Tremmel M, Ma D, Mocco J, Hopkins LN, et al. Hemodynamic-morphologic discriminants for intracranial aneurysm rupture. Stroke. 42:144–152. 2011.
Article38. Xiang J, Tremmel M, Kolega J, Levy EI, Natarajan SK, Meng H. Newtonian viscosity model could overestimate wall shear stress in intracranial aneurysm domes and underestimate rupture risk. J Neurointerv Surg. 4:351–357. 2012.
Article39. Zeng Z, Kallmes DF, Durka M, Ding Y, Lewis D, Kadirvel R, et al. Hemodynamics and anatomy of elastase-induced rabbit aneurysm models: similarity to human cerebral aneurysms? AJNR Am J Neuroradiol. 32:595–601. 2011.
Article40. Zhang Y, Jing L, Zhang Y, Liu J, Yang X. Low wall shear stress is associated with the rupture of intracranial aneurysm with known rupture point: case report and literature review. BMC Neurol. 16:231. 2016.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Considerations of Blood Properties, Outlet Boundary Conditions and Energy Loss Approaches in Computational Fluid Dynamics Modeling
- Wall Shear Stress and Flow Patterns in Unruptured and Ruptured Anterior Communicating Artery Aneurysms Using Computational Fluid Dynamics
- The Current Limitations and Advanced Analysis of Hemodynamic Study of Cerebral Aneurysms
- Computational Fluid Dynamics Modeling in Aortic Diseases
- Angiographic Characteristics of the Intracranial Saccular Aneurysms to Predict the Rupture