J Korean Neurosurg Soc.
2019 Mar;62(2):136-143. 10.3340/jkns.2018.0101.
Hemorrhagic Moyamoya Disease : A Recent Update
- Affiliations
-
- 1Department of Neurosurgery, Kohnan Hospital, Sendai, Japan. fujimur417@kohnan-sendai.or.jp
- 2Department of Neurosurgery, Tohoku University, Sendai, Japan.
- KMID: 2441557
- DOI: http://doi.org/10.3340/jkns.2018.0101
Abstract
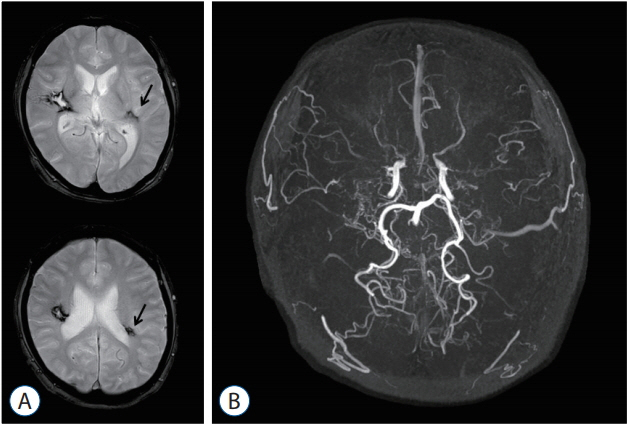
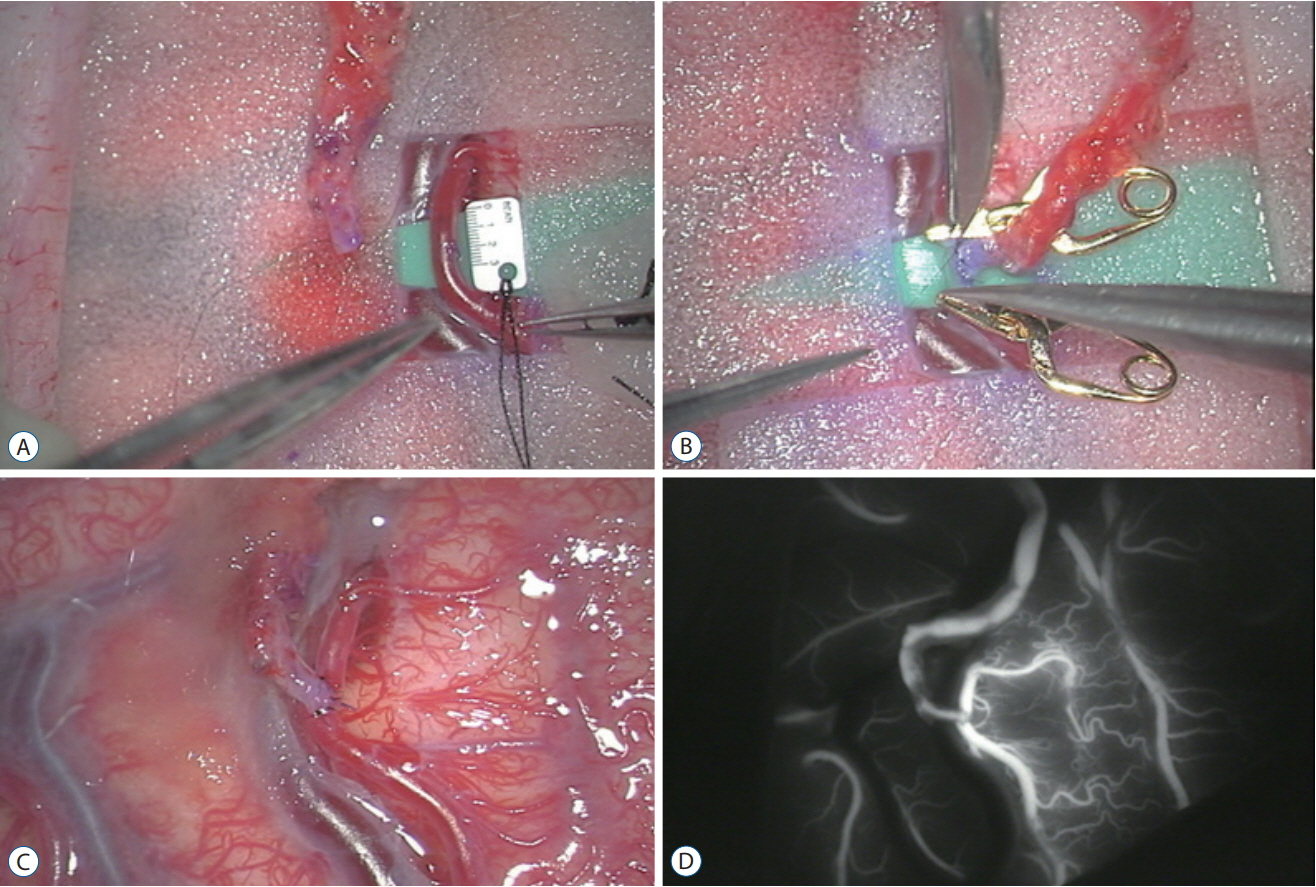
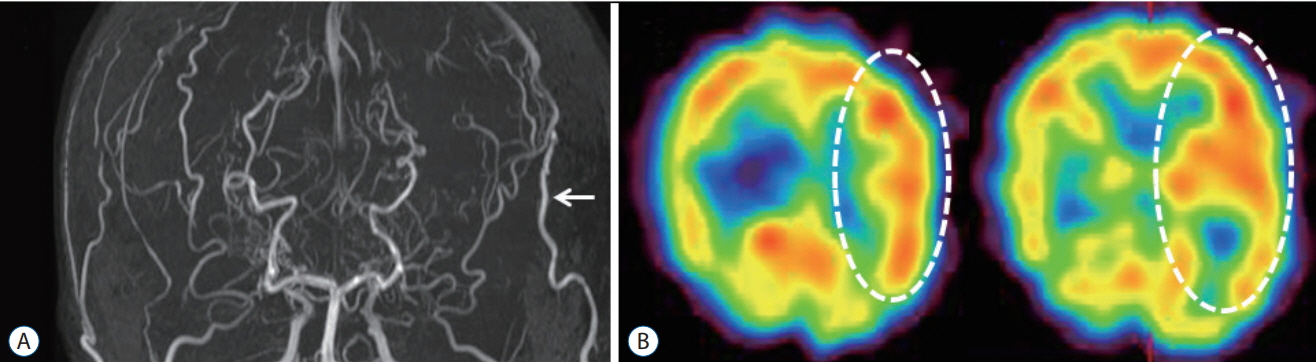
- Moyamoya disease (MMD) is a progressive cerebrovascular disease with unknown etiology, characterized by bilateral stenoocclusive changes at the terminal portion of the internal carotid artery and an abnormal vascular network formation at the base of the brain. MMD has an intrinsic nature to convert the vascular supply for the brain from internal carotid (IC) system to the external carotid (EC) system, as indicated by Suzuki's angiographic staging. Insufficiency of this "˜IC-EC conversion system' could result not only in cerebral ischemia, but also in intracranial hemorrhage from inadequate collateral anastomosis, both of which represent the clinical manifestation of MMD. Surgical revascularization prevents cerebral ischemic attack by improving cerebral blood flow, and recent evidence further suggests that extracranial-intracranial bypass could powerfully reduce the risk of re-bleeding in MMD patients with posterior hemorrhage, who were known to have extremely high re-bleeding risk. Although the exact mechanism underlying the hemorrhagic presentation in MMD is undetermined, most recent angiographic analysis revealed the characteristic angio-architecture related to high re-bleeding risk, such as the extension and dilatation of choroidal collaterals and posterior cerebral artery involvement. We sought to update the current management strategy for hemorrhagic MMD, including the outcome of surgical revascularization for hemorrhagic MMD in our institute. Further investigations will clarify the optimal surgical strategy to prevent hemorrhagic manifestation in patients with MMD.
MeSH Terms
Figure
Reference
-
References
1. Fujimura M, Funaki T, Houkin K, Takahashi JC, Kuroda S, Tomata Y, et al. Intrinsic development of choroidal and thalamic collaterals in hemorrhagic-onset moyamoya disease: case-control study of the Japan adult moyamoya trial. J Neurosurg. 2018; [Epub ahead of print].
Article2. Fujimura M, Inoue T, Shimizu H, Saito A, Mugikura S, Tominaga T. Efficacy of prophylactic blood pressure lowering according to a standardized postoperative management protocol to prevent symptomatic cerebral hyperperfusion after direct revascularization surgery for moyamoya disease. Cerebrovasc Dis. 33:436–445. 2012.
Article3. Fujimura M, Kaneta T, Mugikura S, Shimizu H, Tominaga T. Temporary neurologic deterioration due to cerebral hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in patients with adult-onset moyamoya disease. Surg Neurol. 67:273–282. 2007.
Article4. Fujimura M, Kaneta T, Shimizu H, Tominaga T. Cerebral ischemia owing to compression of the brain by swollen temporal muscle used for encephalo-myo-synangiosis in moyamoya disease. Neurosurg Rev. 32:245–249. 2009.
Article5. Fujimura M, Mugikura S, Kaneta T, Shimizu H, Tominaga T. Incidence and risk factors for symptomatic cerebral hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in patients with moyamoya disease. Surg Neurol. 71:442–447. 2009.
Article6. Fujimura M, Niizuma K, Inoue T, Sato K, Endo H, Shimizu H, et al. Minocycline prevents focal neurologic deterioration due to cerebral hyperperfusion after extracranial-intracranial bypass for moyamoya disease. Neurosurgery. 74:163–170. discussion 170. 2014.
Article7. Fujimura M, Shimizu H, Inoue T, Mugikura S, Saito A, Tominaga T. Significance of focal cerebral hyperperfusion as a cause of transient neurologic deterioration after extracranial-intracranial bypass for moyamoya disease: comparative study with non-moyamoya patients using Nisopropyl-p-[(123)I]iodoamphetamine single-photon emission computed tomography. Neurosurgery. 68:957–964. 2011.
Article8. Fujimura M, Shimizu H, Mugikura S, Tominaga T. Delayed intracerebral hemorrhage after superficial temporal artery-middle cerebral artery anastomosis in a patient with moyamoya disease: possible involvement of cerebral hyperperfusion and increased vascular permeability. Surg Neurol. 71:223–227. 2009.
Article9. Fujimura M, Sonobe S, Nishijima Y, Niizuma K, Sakata H, Kure S, et al. Genetics and biomarkers of moyamoya disease: significance of RNF213 as a susceptibility gene. J Stroke. 16:65–72. 2014.
Article10. Fujimura M, Tominaga T. Current status of revascularization surgery for moyamoya disease: special consideration for its ‘internal carotidexternal carotid (IC-EC) conversion’ as the physiological reorganization system. Tohoku J Exp Med. 236:45–53. 2015.
Article11. Fujimura M, Tominaga T. Lessons learned from moyamoya disease: outcome of direct/indirect revascularization surgery for 150 affected hemispheres. Neurol Med Chir (Tokyo). 52:327–332. 2012.
Article12. Fujimura M, Watanabe M, Narisawa A, Shimizu H, Tominaga T. Increased expression of serum matrix metalloproteinase-9 in patients with moyamoya disease. Surg Neurol. 72:476–480. 2009.
Article13. Funaki T, Takahashi JC, Houkin K, Kuroda S, Takeuchi S, Fujimura M, et al. Angiographic features of hemorrhagic moyamoya disease with high recurrence risk: a supplementary analysis of the Japan adult moyamoya trial. J Neurosurg. 128:777–784. 2018.
Article14. Funaki T, Takahashi JC, Houkin K, Kuroda S, Takeuchi S, Fujimura M, et al. High rebleeding risk associated with choroidal collateral vessels in hemorrhagic moyamoya disease: analysis of a nonsurgical cohort in the Japan adult moyamoya trial. J Neurosurg. 2018; [Epub ahead of print].
Article15. Funaki T, Takahashi JC, Yoshida K, Takagi Y, Fushimi Y, Kikuchi T, et al. Periventricular anastomosis in moyamoya disease: detecting fragile collateral vessels with MR angiography. J Neurosurg. 124:1766–1772. 2016.
Article16. Hayashi K, Horie N, Izumo T, Nagata I. A nationwide survey on unilateral moyamoya disease in Japan. Clin Neurol Neurosurg. 124:1–5. 2014.
Article17. Hayashi T, Shirane R, Fujimura M, Tominaga T. Postoperative neurological deterioration in pediatric moyamoya disease: watershed shift and hyperperfusion. J Neurosurg Pediatr. 6:73–81. 2010.
Article18. Houkin K, Ishikawa T, Yoshimoto T, Abe H. Direct and indirect revascularization for moyamoya disease surgical techniques and peri-operative complications. Clin Neurol Neurosurg 99 Suppl. 2:S142–S145. 1997.
Article19. Hwang JW, Yang HM, Lee H, Lee HK, Jeon YT, Kim JE, et al. Predictive factors of symptomatic cerebral hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in adult patients with moyamoya disease. Br J Anaesth. 110:773–779. 2013.
Article20. Irikura K, Miyasaka Y, Kurata A, Tanaka R, Fujii K, Yada K, et al. A source of haemorrhage in adult patients with moyamoya disease: the significance of tributaries from the choroidal artery. Acta Neurochir (Wien). 138:1282–1286. 1996.
Article21. Kaku Y, Morioka M, Ohmori Y, Kawano T, Kai Y, Fukuoka H, et al. Outer-diameter narrowing of the internal carotid and middle cerebral arteries in moyamoya disease detected on 3D constructive interference in steady-state MR image: is arterial constrictive remodeling a major pathogenesis? Acta Neurochir (Wien). 154:2151–2157. 2012.
Article22. Kamada F, Aoki Y, Narisawa A, Abe Y, Komatsuzaki S, Kikuchi A, et al. A genome-wide association study identifies RNF213 as the first moyamoya disease gene. J Hum Genet. 56:34–40. 2011.
Article23. Kang HS, Kim JH, Phi JH, Kim YY, Kim JE, Wang KC, et al. Plasma matrix metalloproteinases, cytokines and angiogenic factors in moyamoya disease. J Neurol Neurosurg Psychiatry. 81:673–678. 2010.
Article24. Kim JE, Oh CW, Kwon OK, Park SQ, Kim SE, Kim YK. Transient hyperperfusion after superficial temporal artery/middle cerebral artery bypass surgery as a possible cause of postoperative transient neurological deterioration. Cerebrovasc Dis. 25:580–586. 2008.
Article25. Kuroda S, Hashimoto N, Yoshimoto T, Iwasaki Y; Research Committee on Moyamoya Disease in Japan. Radiological findings, clinical course, and outcome in asymptomatic moyamoya disease: results of multicenter survey in Japan. Stroke. 38:1430–1435. 2007.
Article26. Kuroda S, Ishikawa T, Houkin K, Nanba R, Hokari M, Iwasaki Y. Incidence and clinical features of disease progression in adult moyamoya disease. Stroke. 36:2148–2153. 2005.
Article27. Kuroda S, Kashiwazaki D, Akioka N, Koh M, Hori E, Nishikata M, et al. Specific shrinkage of carotid forks in moyamoya disease: a novel key finding for diagnosis. Neurol Med Chir (Tokyo). 55:796–804. 2015.
Article28. Miyamoto S, Yoshimoto T, Hashimoto N, Okada Y, Tsuji I, Tominaga T, et al. Effects of extracranial-intracranial bypass for patients with hemorrhagic moyamoya disease: results of the japan adult moyamoya trial. Stroke. 45:1415–1421. 2014.
Article29. Morioka M, Hamada J, Kawano T, Todaka T, Yano S, Kai Y, et al. Angiographic dilatation and branch extension of the anterior choroidal and posterior communicating arteries are predictors of hemorrhage in adult moyamoya patients. Stroke. 34:90–95. 2003.
Article30. Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis. Health Labour Sciences Research Grant for Research on Measures for Intractable Diseases : Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol Med Chir (Tokyo). 52:245–266. 2012.31. Ryoo S, Cha J, Kim SJ, Choi JW, Ki CS, Kim KH, et al. High-resolution magnetic resonance wall imaging findings of moyamoya disease. Stroke. 45:2457–2460. 2014.
Article32. Sakata H, Fujimura M, Mugikura S, Sato K, Tominaga T. Local vasogenic edema without cerebral hyperperfusion after direct revascularization surgery for moyamoya disease. J Stroke Cerebrovasc Dis. 24:e179–e184. 2015.
Article33. Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 20:288–299. 1969.34. Takahashi JC, Funaki T, Houkin K, Inoue T, Ogasawara K, Nakagawara J, et al. Significance of the hemorrhagic site for recurrent bleeding: prespecified analysis in the Japan adult moyamoya trial. Stroke. 47:37–43. 2016.35. Tashiro R, Fujimura M, Mugikura S, Niizuma K, Endo H, Endo T, et al. Paradoxical association of symptomatic local vasogenic edema with global cerebral hypoperfusion after direct revascularization surgery for adult moyamoya disease. J Stroke Cerebrlvasc Dis. 2018; [Epub ahead of print].
Article36. Tominaga T, Suzuki N, Miyamoto S, Koizumi A, Kuroda S, Takahashi JC, et al. Recommendations for the management of moyamoya disease: a statement from research committee on spontaneous occlusion of the circle of Willis (moyamoya disease) [2nd Edition]. Surg Cereb Stroke. 46:1–24. 2018.
Article37. Tu XK, Fujimura M, Rashad S, Mugikura S, Sakata H, Niizuma K, et al. Uneven cerebral hemodynamic change as a cause of neurological deterioration in the acute stage after direct revascularization for moyamoya disease: cerebral hyperperfusion and remote ischemia caused by the ‘watershed shift’. Neurosurg Rev. 40:507–512. 2017.
Article38. Uchino H, Kuroda S, Hirata K, Shiga T, Houkin K, Tamaki N. Predictors and clinical features of postoperative hyperperfusion after surgical revascularization for moyamoya disease: a serial single photon emission CT/positron emission tomography study. Stroke. 43:2610–2616. 2012.
Article39. Uchino H, Nakayama N, Kazumata K, Kuroda S, Houkin K. Edaravone reduces hyperperfusion-related neurological deficits in adult moyamoya disease: historical control study. Stroke. 47:1930–1932. 2016.
Article40. Yuan M, Liu ZQ, Wang ZQ, Li B, Xu LJ, Xiao XL. High-resolution MR imaging of the arterial wall in moyamoya disease. Neurosci Lett. 584:77–82. 2015.
Article