Korean Circ J.
2019 Apr;49(4):353-360. 10.4070/kcj.2018.0281.
Acute Hemodynamic Changes after Single Administration of Udenafil in Pulmonary Arterial Hypertension: a Phase IIa Study
- Affiliations
-
- 1Division of Cardiology, Department of Medicine, Heart Vascular Stroke Institute, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. dukkyung.kim@gmail.com
- 2Division of Cardiology, Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea.
- 3Department of Cardiology, Yonsei Severance Hospital, Seoul, Korea.
- KMID: 2441139
- DOI: http://doi.org/10.4070/kcj.2018.0281
Abstract
- BACKGROUND AND OBJECTIVES
Udenafil, a new phosphodiesterase-5 inhibitor (PDE5i), has been used to treat erectile dysfunction. Given the proven benefit of PDE5i in pulmonary arterial hypertension (PAH), we evaluated serial hemodynamic changes after single udenafil administration to determine the appropriate therapeutic dose.
METHODS
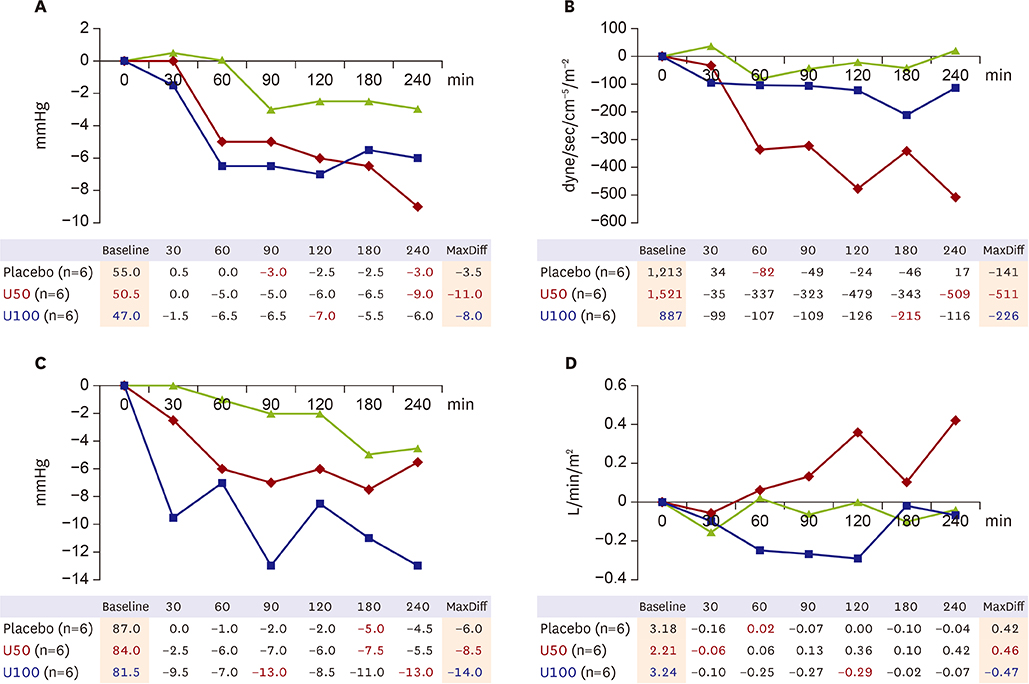
Eighteen patients were randomly allocated into one of 3 groups: placebo, udenafil 50 mg (U50), and udenafil 100 mg (U100). Diagnosis for inclusion was idiopathic PAH or PAH associated with connective tissue disease. Patients with any contraindication to PDE5i, and/or PDE5i treatment in the past 1 month were excluded. Continuous hemodynamic monitoring was performed by placing a Swan-Ganz catheter. Information on cardiac index (CI), mean pulmonary arterial pressure (mPAP), mean systemic arterial pressure (mSAP), pulmonary arterial wedge pressure (PAWP), and pulmonary vascular resistance index (PVRI) was obtained for 4 hours after drug administration.
RESULTS
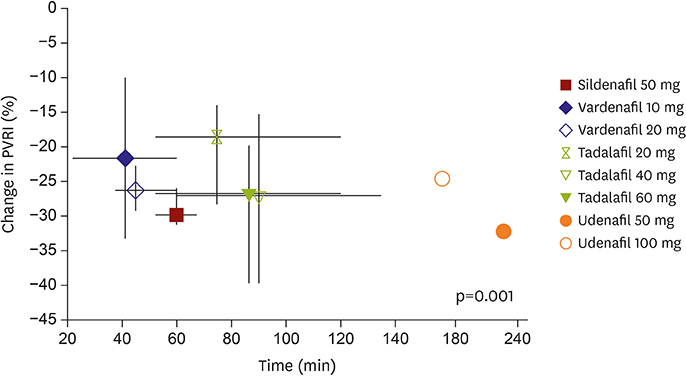
The mPAP significantly decreased in both the U50 and U100 (−11 mmHg and −8 mmHg from baseline, respectively, p < 0.1). The mSAP also decreased in both U50 and U100; however, the decrease was greater in the U100 (Δ=−8.5 mmHg and Δ=−14.0 mmHg). CI increased in the U50, but decreased in the U100. Although PVRI decreased in both, statistical significance was only achieved in the U50 compared to placebo. PAWP was stable during monitoring. U50 had at least 4 hour-effect after administration. Only 2 patients with U100 experienced mild adverse events.
CONCLUSIONS
This is the first demonstration of the acute hemodynamic changes induced by udenafil. U50 is considered an optimal dose for treating PAH with more than 4-hour treatment effect. TRIAL REGISTRATION: ClinicalTrials.gov Identifier: NCT01553721.
MeSH Terms
-
Arterial Pressure
Catheters
Connective Tissue Diseases
Cyclic Nucleotide Phosphodiesterases, Type 5
Diagnosis
Erectile Dysfunction
Hemodynamics*
Humans
Hypertension*
Hypertension, Pulmonary
Male
Phosphodiesterase 5 Inhibitors
Pulmonary Wedge Pressure
Vascular Resistance
Cyclic Nucleotide Phosphodiesterases, Type 5
Phosphodiesterase 5 Inhibitors
Figure
Cited by 1 articles
-
Udenafil as a Therapeutic Option for Pulmonary Arterial Hypertension
Hyung Yoon Kim, Kye Hun Kim
Korean Circ J. 2019;49(4):361-362. doi: 10.4070/kcj.2019.0023.
Reference
-
1. Zhao C, Kim SW, Yang DY, et al. Efficacy and safety of once-daily dosing of udenafil in the treatment of erectile dysfunction: results of a multicenter, randomized, double-blind, placebo-controlled trial. Eur Urol. 2011; 60:380–387.
Article2. Park HJ, Park JK, Park K, Min K, Park NC. Efficacy of udenafil for the treatment of erectile dysfunction up to 12 hours after dosing: a randomized placebo-controlled trial. J Sex Med. 2010; 7:2209–2216.
Article3. Kim BH, Lim HS, Chung JY, et al. Safety, tolerability and pharmacokinetics of udenafil, a novel PDE-5 inhibitor, in healthy young Korean subjects. Br J Clin Pharmacol. 2008; 65:848–854.
Article4. Kim HL, Kim YJ, Kim KH, et al. Therapeutic effects of udenafil on pressure-overload cardiac hypertrophy. Hypertens Res. 2015; 38:597–604.
Article5. Kim KH, Kim HK, Hwang IC, et al. PDE 5 inhibition with udenafil improves left ventricular systolic/diastolic functions and exercise capacity in patients with chronic heart failure with reduced ejection fraction; a 12-week, randomized, double-blind, placebo-controlled trial. Am Heart J. 2015; 169:813–822.e3.
Article6. Kreisel W, Deibert P, Kupcinskas L, et al. The phosphodiesterase-5-inhibitor udenafil lowers portal pressure in compensated preascitic liver cirrhosis. A dose-finding phase-II-study. Dig Liver Dis. 2015; 47:144–150.
Article7. Galiè N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005; 353:2148–2157.
Article8. Galiè N, Brundage BH, Ghofrani HA, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009; 119:2894–2903.
Article9. Ghofrani HA, Voswinckel R, Reichenberger F, et al. Differences in hemodynamic and oxygenation responses to three different phosphodiesterase-5 inhibitors in patients with pulmonary arterial hypertension: a randomized prospective study. J Am Coll Cardiol. 2004; 44:1488–1496.10. Paick JS, Kim SW, Yang DY, et al. The efficacy and safety of udenafil, a new selective phosphodiesterase type 5 inhibitor, in patients with erectile dysfunction. J Sex Med. 2008; 5:946–953.
Article11. Salem EA, Kendirci M, Hellstrom WJ. Udenafil, a long-acting PDE5 inhibitor for erectile dysfunction. Curr Opin Investig Drugs. 2006; 7:661–669.12. Kim TE, Kim BH, Kim JR, et al. Effect of food on the pharmacokinetics of the oral phosphodiesterase 5 inhibitor udenafil for the treatment of erectile dysfunction. Br J Clin Pharmacol. 2009; 68:43–46.
Article13. Fitzgerald AA, Powers JD, Ho PM, et al. Impact of medication nonadherence on hospitalizations and mortality in heart failure. J Card Fail. 2011; 17:664–669.
Article14. Frishman WH. Importance of medication adherence in cardiovascular disease and the value of once-daily treatment regimens. Cardiol Rev. 2007; 15:257–263.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Updated clinical classification of pulmonary hypertension
- Acute Hemodynamic Effects of IV Nitroglycerin in the Patients with Chronic Pulmonary Hypertension
- Udenafil as a Therapeutic Option for Pulmonary Arterial Hypertension
- Pulmonary Arterial Hypertension
- A Case of Pulmonary Arterial Hypertension Associated With Hyperthyroidism, Persistent After Euthyroidism Was Obtained