Ann Rehabil Med.
2019 Feb;43(1):62-73. 10.5535/arm.2019.43.1.62.
Repetitive Transcranial Magnetic Stimulation Enhances Recovery in Central Cord Syndrome Patients
- Affiliations
-
- 1Department of Rehabilitation Medicine, Dankook University College of Medicine, Cheonan, Korea. rhhyun@dankook.ac.kr
- 2Department of Nanobiomedical Science and BK21 Plus NBM Global Research Center for Regenerative Medicine, Dankook University, Cheonan, Korea.
- 3Institute of Tissue Regeneration Engineering (ITREN), Dankook University, Cheonan, Korea.
- 4Wiregene Co. Ltd., Cheonan, Korea.
- KMID: 2440953
- DOI: http://doi.org/10.5535/arm.2019.43.1.62
Abstract
OBJECTIVE
To investigate the effect of repetitive transcranial magnetic stimulation (rTMS) on neurological and functional recovery in patients with central cord syndrome (CCS) involving the upper extremities between the treated and non-treated sides of the treated group and whether the outcomes are comparable to that of the untreated control group.
METHODS
Nineteen CCS patients were treated with high-frequency (20 Hz) rTMS over the motor cortex for 5 days. The stimulation side was randomly selected, and all the subjects received conventional occupational therapy during the rTMS-treatment period. Twenty CCS patients who did not receive rTMS were considered as controls. Clinical assessments, including those by the International Standard for Neurological Classification of Spinal Cord Injury, the Jebsen-Taylor Hand Function Test, and the O'Connor Finger Dexterity Test were performed initially and followed up for 1 month after rTMS treatment or 5 weeks after initial assessments.
RESULTS
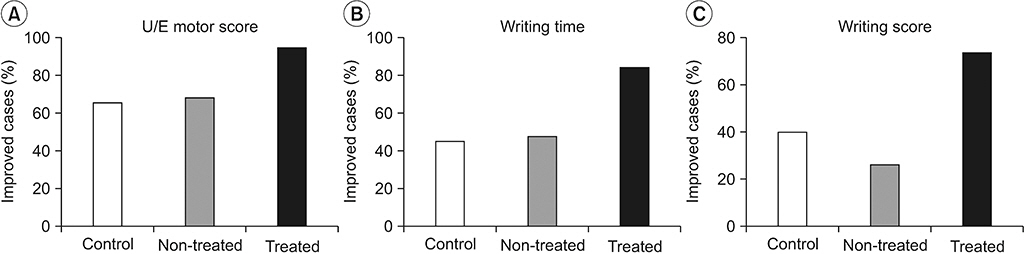
The motor scores for upper extremities were increased and the number of improved cases was greater for the treated side in rTMS-treated patients than for the non-treated side in rTMS-treated patients or controls. The improved cases for writing time and score measured on the Jebsen-Taylor Hand Function Test were also significantly greater in number on the rTMS-treated side compared with the non-treated side and controls. There were no adverse effects during rTMS therapy or the follow-up period.
CONCLUSION
The results of the application of high-frequency rTMS treatment to CCS patients suggest that rTMS can enhance the motor recovery and functional fine motor task performance of the upper extremities in such individuals.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Effects of Combined Upper Limb Robotic Therapy in Patients With Tetraplegic Spinal Cord Injury
Joo Hwan Jung, Hye Jin Lee, Duk Youn Cho, Jung-Eun Lim, Bum Suk Lee, Seung Hyun Kwon, Hae Young Kim, Su Jeong Lee
Ann Rehabil Med. 2019;43(4):445-457. doi: 10.5535/arm.2019.43.4.445.
Reference
-
1. Ahuja CS, Fehlings M. Concise Review: Bridging the gap: novel neuroregenerative and neuroprotective strategies in spinal cord injury. Stem Cells Transl Med. 2016; 5:914–24.
Article2. Nagoshi N, Okano H. Applications of induced pluripotent stem cell technologies in spinal cord injury. J Neurochem. 2017; 141:848–60.
Article3. Hong JY, Lee SH, Lee SC, Kim JW, Kim KP, Kim SM, et al. Therapeutic potential of induced neural stem cells for spinal cord injury. J Biol Chem. 2014; 289:32512–25.
Article4. Harvey AR, Lovett SJ, Majda BT, Yoon JH, Wheeler LP, Hodgetts SI. Neurotrophic factors for spinal cord repair: which, where, how and when to apply, and for what period of time? Brain Res. 2015; 1619:36–71.
Article5. Joo NY, Knowles JC, Lee GS, Kim JW, Kim HW, Son YJ, et al. Effects of phosphate glass fiber-collagen scaffolds on functional recovery of completely transected rat spinal cords. Acta Biomater. 2012; 8:1802–12.
Article6. Fuhrmann T, Anandakumaran PN, Shoichet MS. Combinatorial therapies after spinal cord injury: how can biomaterials help? Adv Healthc Mater. 2017; 6:1601130.7. Hollis ER 2nd, Ishiko N, Yu T, Lu CC, Haimovich A, Tolentino K, et al. Ryk controls remapping of motor cortex during functional recovery after spinal cord injury. Nat Neurosci. 2016; 19:697–705.
Article8. Filli L, Engmann AK, Zorner B, Weinmann O, Moraitis T, Gullo M, et al. Bridging the gap: a reticulo-propriospinal detour bypassing an incomplete spinal cord injury. J Neurosci. 2014; 34:13399–410.
Article9. Liu ZH, Yip PK, Adams L, Davies M, Lee JW, Michael GJ, et al. A single bolus of docosahexaenoic acid promotes neuroplastic changes in the innervation of spinal cord interneurons and motor neurons and improves functional recovery after spinal cord injury. J Neurosci. 2015; 35:12733–52.
Article10. Siegel CS, Fink KL, Strittmatter SM, Cafferty WB. Plasticity of intact rubral projections mediates spontaneous recovery of function after corticospinal tract injury. J Neurosci. 2015; 35:1443–57.
Article11. Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007; 55:187–99.
Article12. Ellaway PH, Vasquez N, Craggs M. Induction of central nervous system plasticity by repetitive transcranial magnetic stimulation to promote sensorimotor recovery in incomplete spinal cord injury. Front Integr Neurosci. 2014; 8:42.
Article13. Jette F, Cote I, Meziane HB, Mercier C. Effect of singlesession repetitive transcranial magnetic stimulation applied over the hand versus leg motor area on pain after spinal cord injury. Neurorehabil Neural Repair. 2013; 27:636–43.
Article14. Kumru H, Murillo N, Samso JV, Valls-Sole J, Edwards D, Pelayo R, et al. Reduction of spasticity with repetitive transcranial magnetic stimulation in patients with spinal cord injury. Neurorehabil Neural Repair. 2010; 24:435–41.
Article15. Boldt I, Eriks-Hoogland I, Brinkhof MW, de Bie R, Joggi D, von Elm E, et al. Non-pharmacological interventions for chronic pain in people with spinal cord injury. Cochrane Database Syst Rev. 2014; CD009177.
Article16. McKinley W, Santos K, Meade M, Brooke K. Incidence and outcomes of spinal cord injury clinical syndromes. J Spinal Cord Med. 2007; 30:215–24.
Article17. van Middendorp JJ, Pouw MH, Hayes KC, Williams R, Chhabra HS, Putz C, et al. Diagnostic criteria of traumatic central cord syndrome. Part 2. A questionnaire survey among spine specialists. Spinal Cord. 2010; 48:657–63.
Article18. Fehlings MG, Tetreault L, Nater A, Choma T, Harrop J, Mroz T, et al. The aging of the global population: the changing epidemiology of disease and spinal disorders. Neurosurgery. 2015; 77 Suppl 4:S1–5.19. Schneider RC, Cherry G, Pantek H. The syndrome of acute central cervical spinal cord injury; with special reference to the mechanisms involved in hyperextension injuries of cervical spine. J Neurosurg. 1954; 11:546–77.20. Mathiowetz V, Weber K, Volland G, Kashman N. Reliability and validity of grip and pinch strength evaluations. J Hand Surg Am. 1984; 9:222–6.
Article21. Oudega M, Perez MA. Corticospinal reorganization after spinal cord injury. J Physiol. 2012; 590:3647–63.
Article22. Nudo RJ. Rehabilitation: boost for movement. Nature. 2015; 527:314–5.23. Fouad K, Tetzlaff W. Rehabilitative training and plasticity following spinal cord injury. Exp Neurol. 2012; 235:91–9.
Article24. Girgis J, Merrett D, Kirkland S, Metz GA, Verge V, Fouad K. Reaching training in rats with spinal cord injury promotes plasticity and task specific recovery. Brain. 2007; 130:2993–3003.
Article25. Fujiwara T, Tsuji T, Honaga K, Hase K, Ushiba J, Liu M. Transcranial direct current stimulation modulates the spinal plasticity induced with patterned electrical stimulation. Clin Neurophysiol. 2011; 122:1834–7.
Article26. Hou J, Nelson R, Nissim N, Parmer R, Thompson FJ, Bose P. Effect of combined treadmill training and magnetic stimulation on spasticity and gait impairments after cervical spinal cord injury. J Neurotrauma. 2014; 31:1088–106.
Article27. Massie CL, Tracy BL, Malcolm MP. Functional repetitive transcranial magnetic stimulation increases motor cortex excitability in survivors of stroke. Clin Neurophysiol. 2013; 124:371–8.
Article28. Kim SY, Shin SB, Lee SJ, Kim TU, Hyun JK. Factors associated with upper extremity functional recovery following low-frequency repetitive transcranial magnetic stimulation in stroke patients. Ann Rehabil Med. 2016; 40:373–82.
Article29. Brys M, Fox MD, Agarwal S, Biagioni M, Dacpano G, Kumar P, et al. Multifocal repetitive TMS for motor and mood symptoms of Parkinson disease: A randomized trial. Neurology. 2016; 87:1907–15.30. Elzamarany E, Afifi L, El-Fayoumy NM, Salah H, Nada M. Motor cortex rTMS improves dexterity in relapsing-remitting and secondary progressive multiple sclerosis. Acta Neurol Belg. 2016; 116:145–50.
Article31. Kumru H, Benito-Penalva J, Valls-Sole J, Murillo N, Tormos JM, Flores C, et al. Placebo-controlled study of rTMS combined with Lokomat gait training for treatment in subjects with motor incomplete spinal cord injury. Exp Brain Res. 2016; 234:3447–55.
Article32. Kuppuswamy A, Balasubramaniam AV, Maksimovic R, Mathias CJ, Gall A, Craggs MD, et al. Action of 5 Hz repetitive transcranial magnetic stimulation on sensory, motor and autonomic function in human spinal cord injury. Clin Neurophysiol. 2011; 122:2452–61.33. Belci M, Catley M, Husain M, Frankel HL, Davey NJ. Magnetic brain stimulation can improve clinical outcome in incomplete spinal cord injured patients. Spinal Cord. 2004; 42:417–9.
Article34. Lefaucheur JP, Andre-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol. 2014; 125:2150–206.
Article35. Chervyakov AV, Chernyavsky AY, Sinitsyn DO, Piradov MA. Possible mechanisms underlying the therapeutic effects of transcranial magnetic stimulation. Front Hum Neurosci. 2015; 9:303.
Article36. Soundara Rajan T, Ghilardi MFM, Wang HY, Mazzon E, Bramanti P, Restivo D, et al. Mechanism of action for rTMS: a working hypothesis based on animal studies. Front Physiol. 2017; 8:457.
Article37. Vlachos A, Muller-Dahlhaus F, Rosskopp J, Lenz M, Ziemann U, Deller T. Repetitive magnetic stimulation induces functional and structural plasticity of excitatory postsynapses in mouse organotypic hippocampal slice cultures. J Neurosci. 2012; 32:17514–23.
Article38. Sasaki N, Mizutani S, Kakuda W, Abo M. Comparison of the effects of high- and low-frequency repetitive transcranial magnetic stimulation on upper limb hemiparesis in the early phase of stroke. J Stroke Cerebrovasc Dis. 2013; 22:413–8.
Article39. Noskin O, Krakauer JW, Lazar RM, Festa JR, Handy C, O’Brien KA, et al. Ipsilateral motor dysfunction from unilateral stroke: implications for the functional neuroanatomy of hemiparesis. J Neurol Neurosurg Psychiatry. 2008; 79:401–6.
Article40. Rossi S, Hallett M, Rossini PM, Pascual-Leone A; Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009; 120:2008–39.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Repetitive Transcranial Magnetic Stimulation for Wernicke-Korsakoff Syndrome: A Case Report
- Repetitive Transcranial Magnetic Stimulation for Limb-Kinetic Apraxia in Parkinson's Disease
- Application of Non-invasive Brain Stimulation on Dysphagia after Stroke
- Stroke Update 2011: Stroke Rehabilitation
- Predictors of Response to Repetitive Transcranial Magnetic Stimulation in Depression: A Review of Recent Updates