J Periodontal Implant Sci.
2019 Feb;49(1):25-38. 10.5051/jpis.2019.49.1.25.
Bone healing dynamics associated with 3 implants with different surfaces: histologic and histomorphometric analyses in dogs
- Affiliations
-
- 1Department of Periodontics, One-Stop Specialty Center, Seoul National University Dental Hospital, Seoul, Korea.
- 2Department of Periodontology, Seoul National University School of Dentistry, Seoul, Korea. periokoo@snu.ac.kr, ymlee@snu.ac.kr
- 3Department of Prosthodontics, Seoul National University School of Dentistry, Seoul, Korea.
- 4Dental Research Institute, Seoul National University School of Dentistry, Seoul, Korea.
- 5Department of Health Policy and Management, Korea University College of Health Science, Seoul, Korea.
- KMID: 2440175
- DOI: http://doi.org/10.5051/jpis.2019.49.1.25
Abstract
- PURPOSE
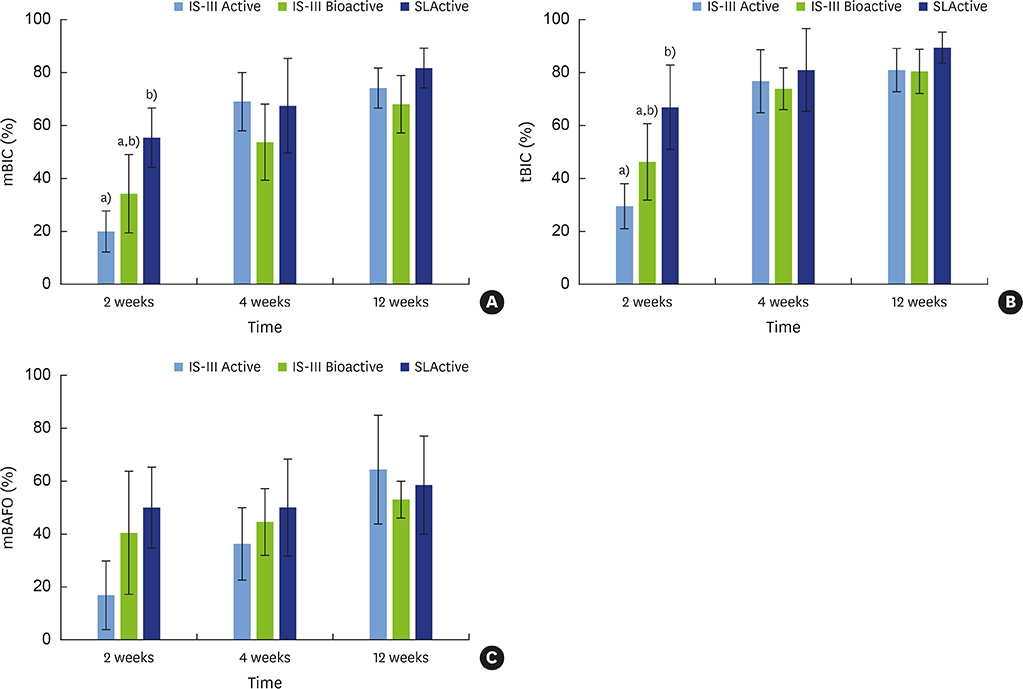
This study evaluated differences in bone healing and remodeling among 3 implants with different surfaces: sandblasting and large-grit acid etching (SLA; IS-III Active®), SLA with hydroxyapatite nanocoating (IS-III Bioactive®), and SLA stored in sodium chloride solution (SLActive®).
METHODS
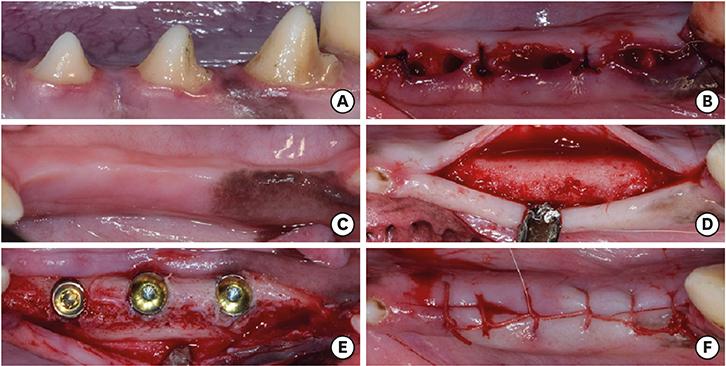
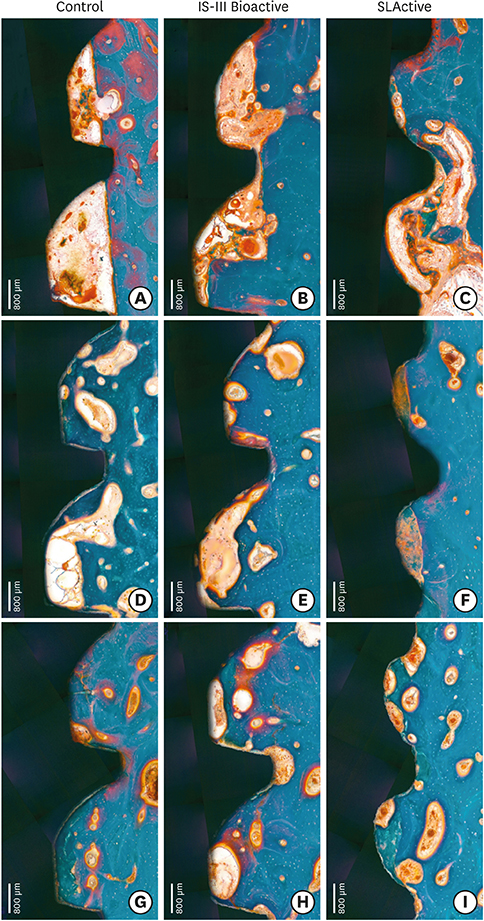
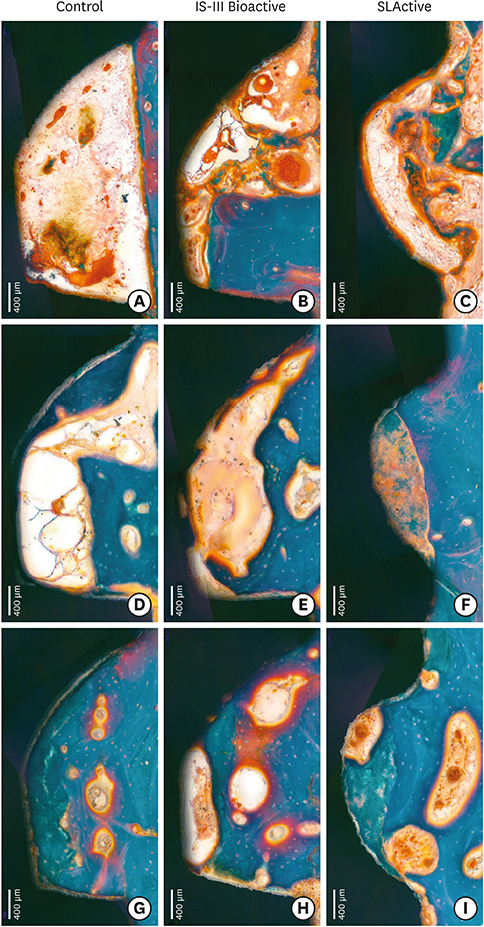
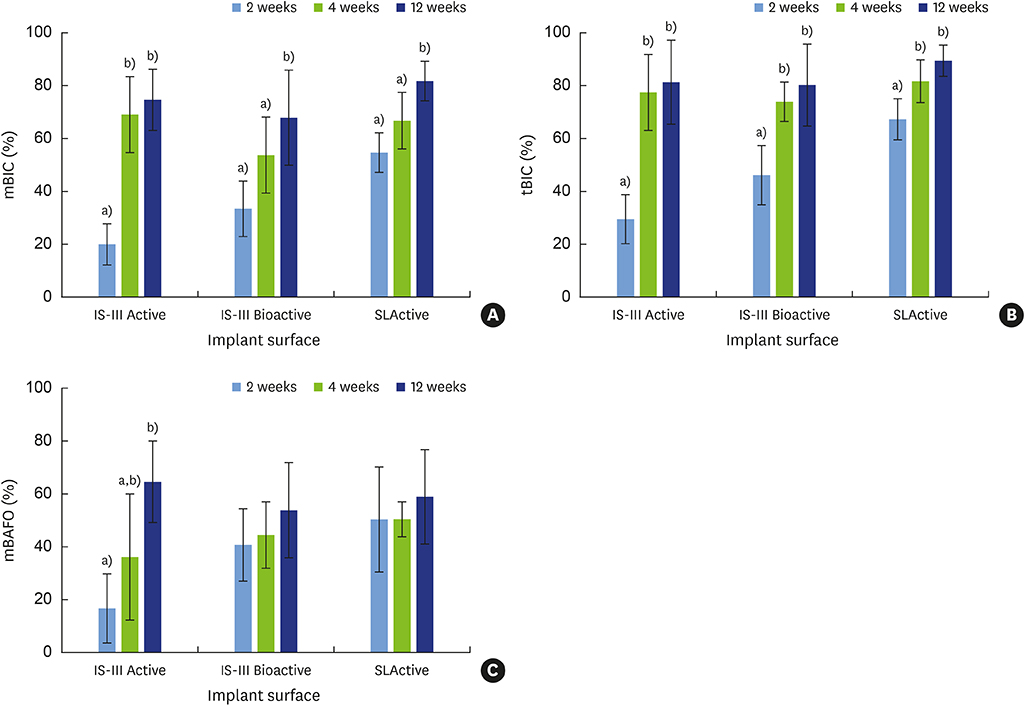
The mandibular second, third, and fourth premolars of 9 dogs were extracted. After 4 weeks, 9 dogs with edentulous alveolar ridges underwent surgical placement of 3 implants bilaterally and were allowed to heal for 2, 4, or 12 weeks. Histologic and histomorphometric analyses were performed on 54 stained slides based on the following parameters: vertical marginal bone loss at the buccal and lingual aspects of the implant (b-MBL and l-MBL, respectively), mineralized bone-to-implant contact (mBIC), osteoid-to-implant contact (OIC), total bone-to-implant contact (tBIC), mineralized bone area fraction occupied (mBAFO), osteoid area fraction occupied (OAFO), and total bone area fraction occupied (tBAFO) in the threads of the region of interest. Two-way analysis of variance (3 types of implant surface×3 healing time periods) and additional analyses for simple effects were performed.
RESULTS
Statistically significant differences were observed across the implant surfaces for OIC, mBIC, tBIC, OAFO, and tBAFO. Statistically significant differences were observed over time for l-MBL, mBIC, tBIC, mBAFO, and tBAFO. In addition, an interaction effect between the implant surface and the healing time period was observed for mBIC, tBIC, and mBAFO.
CONCLUSIONS
Our results suggest that implant surface wettability facilitates bone healing dynamics, which could be attributed to the improvement of early osseointegration. In addition, osteoblasts might become more activated with the use of HA-coated surface implants than with hydrophobic surface implants in the remodeling phase.
Keyword
MeSH Terms
Figure
Reference
-
1. Terheyden H, Lang NP, Bierbaum S, Stadlinger B. Osseointegration--communication of cells. Clin Oral Implants Res. 2012; 23:1127–1135.2. Tomisa AP, Launey ME, Lee JS, Mankani MH, Wegst UG, Saiz E. Nanotechnology approaches to improve dental implants. Int J Oral Maxillofac Implants. 2011; 26:Suppl. 25–44.3. Aljateeli M, Wang HL. Implant microdesigns and their impact on osseointegration. Implant Dent. 2013; 22:127–132.
Article4. Swetha M, Sahithi K, Moorthi A, Srinivasan N, Ramasamy K, Selvamurugan N. Biocomposites containing natural polymers and hydroxyapatite for bone tissue engineering. Int J Biol Macromol. 2010; 47:1–4.
Article5. Ong JL, Chan DC. Hydroxyapatite and their use as coatings in dental implants: a review. Crit Rev Biomed Eng. 2000; 28:667–707.
Article6. Whitehead RY, Lucas LC, Lacefield WR. The effect of dissolution on plasma sprayed hydroxylapatite coatings on titanium. Clin Mater. 1993; 12:31–39.
Article7. Wheeler SL. Eight-year clinical retrospective study of titanium plasma-sprayed and hydroxyapatite-coated cylinder implants. Int J Oral Maxillofac Implants. 1996; 11:340–350.
Article8. Thierer T, Davliakos JP, Keith JD Jr, Sanders JJ, Tarnow DP, Rivers JA. Five-year prospective clinical evaluation of highly crystalline HA MP-1-coated dental implants. J Oral Implantol. 2008; 34:39–46.
Article9. Artzi Z, Carmeli G, Kozlovsky A. A distinguishable observation between survival and success rate outcome of hydroxyapatite-coated implants in 5-10 years in function. Clin Oral Implants Res. 2006; 17:85–93.
Article10. Rupp F, Scheideler L, Olshanska N, de Wild M, Wieland M, Geis-Gerstorfer J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J Biomed Mater Res A. 2006; 76:323–334.
Article11. Buser D, Broggini N, Wieland M, Schenk RK, Denzer AJ, Cochran DL, et al. Enhanced bone apposition to a chemically modified SLA titanium surface. J Dent Res. 2004; 83:529–533.
Article12. Qu Z, Rausch-Fan X, Wieland M, Matejka M, Schedle A. The initial attachment and subsequent behavior regulation of osteoblasts by dental implant surface modification. J Biomed Mater Res A. 2007; 82:658–668.
Article13. Lai HC, Zhuang LF, Liu X, Wieland M, Zhang ZY, Zhang ZY. The influence of surface energy on early adherent events of osteoblast on titanium substrates. J Biomed Mater Res A. 2010; 93:289–296.
Article14. Lang NP, Salvi GE, Huynh-Ba G, Ivanovski S, Donos N, Bosshardt DD. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin Oral Implants Res. 2011; 22:349–356.
Article15. Calciolari E, Mardas N, Dereka X, Anagnostopoulos AK, Tsangaris GT, Donos N. Protein expression during early stages of bone regeneration under hydrophobic and hydrophilic titanium domes. A pilot study. J Periodontal Res. 2018; 53:174–187.
Article16. Matsuo K, Irie N. Osteoclast-osteoblast communication. Arch Biochem Biophys. 2008; 473:201–209.
Article17. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010; 8:e1000412.
Article18. Vlacic-Zischke J, Hamlet SM, Friis T, Tonetti MS, Ivanovski S. The influence of surface microroughness and hydrophilicity of titanium on the up-regulation of TGFβ/BMP signalling in osteoblasts. Biomaterials. 2011; 32:665–671.
Article19. Donath K, Breuner G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Säge-Schliff (sawing and grinding) technique. J Oral Pathol. 1982; 11:318–326.
Article20. Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013; 28:2–17.
Article21. Sato M, Aslani A, Sambito MA, Kalkhoran NM, Slamovich EB, Webster TJ. Nanocrystalline hydroxyapatite/titania coatings on titanium improves osteoblast adhesion. J Biomed Mater Res A. 2008; 84:265–272.
Article22. Sohn SH, Jun HK, Kim CS, Kim KN, Chung SM, Shin SW, et al. Biological responses in osteoblast-like cell line according to thin layer hydroxyapatite coatings on anodized titanium. J Oral Rehabil. 2006; 33:898–911.
Article23. Oates TW, Valderrama P, Bischof M, Nedir R, Jones A, Simpson J, et al. Enhanced implant stability with a chemically modified SLA surface: a randomized pilot study. Int J Oral Maxillofac Implants. 2007; 22:755–760.24. Stadlinger B, Lode AT, Eckelt U, Range U, Schlottig F, Hefti T, et al. Surface-conditioned dental implants: an animal study on bone formation. J Clin Periodontol. 2009; 36:882–891.
Article25. Schenk RK, Buser D. Osseointegration: a reality. Periodontol 2000. 1998; 17:22–35.
Article26. Futami T, Fujii N, Ohnishi H, Taguchi N, Kusakari H, Ohshima H, et al. Tissue response to titanium implants in the rat maxilla: ultrastructural and histochemical observations of the bone-titanium interface. J Periodontol. 2000; 71:287–298.
Article27. Haider R, Watzek G, Plenk H. Effects of drill cooling and bone structure on IMZ implant fixation. Int J Oral Maxillofac Implants. 1993; 8:83–91.28. Buser D, Schenk RK, Steinemann S, Fiorellini JP, Fox CH, Stich H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J Biomed Mater Res. 1991; 25:889–902.
Article29. Ong JL, Carnes DL, Bessho K. Evaluation of titanium plasma-sprayed and plasma-sprayed hydroxyapatite implants in vivo . Biomaterials. 2004; 25:4601–4606.
Article30. Park YS, Yi KY, Lee IS, Han CH, Jung YC. The effects of ion beam-assisted deposition of hydroxyapatite on the grit-blasted surface of endosseous implants in rabbit tibiae. Int J Oral Maxillofac Implants. 2005; 20:31–38.31. Orsini G, Piattelli M, Scarano A, Petrone G, Kenealy J, Piattelli A, et al. Randomized, controlled histologic and histomorphometric evaluation of implants with nanometer-scale calcium phosphate added to the dual acid-etched surface in the human posterior maxilla. J Periodontol. 2007; 78:209–218.
Article32. Mendes VC, Moineddin R, Davies JE. The effect of discrete calcium phosphate nanocrystals on bone-bonding to titanium surfaces. Biomaterials. 2007; 28:4748–4755.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bone response of three different surface implants : Histomorphometric, perio test value and resonance frequency analysis in beagle dogs
- Bone response of three different surface implants: histomorphometric and resonance frequency analysis in dogs
- A study on the stability of 5 different surface treatment methods to dental implant using resonance frequency and histomorphometric analysis
- Histologic evaluation and removal torque analysis of nano- and microtreated titanium implants in the dogs
- A histomorphometric study of dental implants with different surface characteristics