Ann Hepatobiliary Pancreat Surg.
2019 Feb;23(1):56-60. 10.14701/ahbps.2019.23.1.56.
Effect of early and delay starting of enteral feeding in post-pancreaticoduodenectomy patients
- Affiliations
-
- 1Department of Hepatobiliary, Pancreatic & Liver Transplant Surgery, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh. dbidhan@yahoo.com
- KMID: 2439046
- DOI: http://doi.org/10.14701/ahbps.2019.23.1.56
Abstract
- BACKGROUNDS/AIMS
This study was undertaken to see the effect of early starting of enteral feeding after pancreatoduodenectomy (PD). The results were compared with existing nutritional practice in which enteral feeding started, usually after 7 to 8 postoperative day (PODs) in our institute.
METHODS
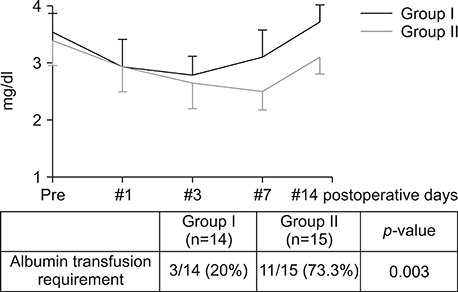
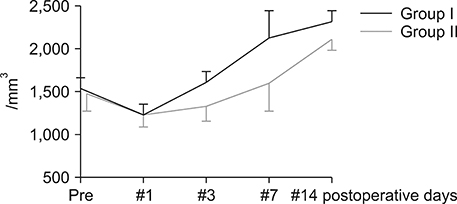
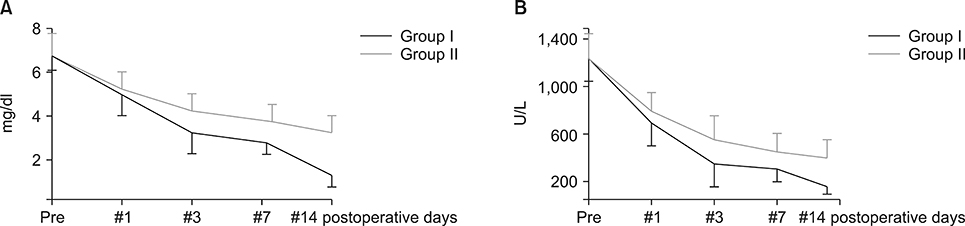
Thirty patients whome underwent a PD from January 2016 to December 2016 were included in the study. They were divided into two groups, I and II. In group I (n=15), enteral feeding was started from the 2(nd) POD through the nasojejunal feeding tube along with parenteral partial nutrition support. In group II (n=15), no enteral feeding was given up to seventh and eighth PODs, except the perenteral feeding. Post-operatively, serum albumin levels, total lymphocyte count, total bilirubin levels, serum alkaline phosphate levels were measured for two weeks postoperatively in all the patients for assessing nutritional, immunological and cholestasis status. The mortality, morbidity and lengths of post-operative hospital stay were also recorded.
RESULTS
Postoperatively, the serum albumin level and lymphocyte count decreased from the pre-operative level on the third POD and it gradually increased from the seventh POD onwards in both groups. However, they remained persistently higher in group I than group II. The total bilirubin and alkaline phosphatase decreased to normal levels within the seventh POD in Group I. However, they remained higher than normal levels on POD 14 in Group II. The morbidity and hospital stay was significantly lower in group I than group II.
CONCLUSIONS
Early enteral feeding should be considered after PD. This is because it will improve nutritional, immunological status and cholestasis. Therefore, it reduces morbidity and shortens the hospital stay.
MeSH Terms
Figure
Reference
-
1. Pappas S, Krzywda E, McDowell N. Nutrition and pancreaticoduodenectomy. Nutr Clin Pract. 2010; 25:234–243.
Article2. Alfargieny R, Bodalal Z, Bendardaf R, El-Fadli M, Langhi S. Nutritional status as a predictive marker for surgical site infection in total joint arthroplasty. Avicenna J Med. 2015; 5:117–122.
Article3. Quain AM, Khardori NM. Nutrition in wound care management: a comprehensive overview. Wounds. 2015; 27:327–335.4. Phillips B. Reducing gastrointestinal anastomotic leak rates: review of challenges and solutions. Open Access Surg. 2016; 9:5–14.
Article5. Nagata S, Fukuzawa K, Iwashita Y, Kabashima A, Kinoshita T, Wakasugi K, et al. Comparison of enteral nutrition with combined enteral and parenteral nutrition in post-pancreaticoduodenectomy patients: a pilot study. Nutr J. 2009; 8:24.
Article6. Worsh CE, Tatarian T, Singh A, Pucci MJ, Winter JM, Yeo CJ, et al. Total parenteral nutrition in patients following pancreaticoduodenectomy: lessons from 1184 patients. J Surg Res. 2017; 218:156–161.
Article7. Baradi H, Walsh RM, Henderson JM, Vogt D, Popovich M. Postoperative jejunal feeding and outcome of pancreaticoduodenectomy. J Gastrointest Surg. 2004; 8:428–433.
Article8. Gerritsen A, Besselink MG, Cieslak KP, Vriens MR, Steenhagen E, van Hillegersberg R, et al. Efficacy and complications of nasojejunal, jejunostomy and parenteral feeding after pancreaticoduodenectomy. J Gastrointest Surg. 2012; 16:1144–1151.
Article9. Gärtner S, Krüger J, Aghdassi AA, Steveling A, Simon P, Lerch MM, et al. Nutrition in pancreatic cancer: a review. Gastrointest Tumors. 2016; 2:195–202.
Article10. Zhu XH, Wu YF, Qiu YD, Jiang CP, Ding YT. Effect of early enteral combined with parenteral nutrition in patients undergoing pancreaticoduodenectomy. World J Gastroenterol. 2013; 19:5889–5896.
Article11. Akbarshahi H, Andersson B, Nordén M, Andersson R. Perioperative nutrition in elective gastrointestinal surgery--potential for improvement? Dig Surg. 2008; 25:165–174.12. Liao Q, Zhao YP, Wang WB, Dai MH, Hu Y, Liu ZW, et al. [Perioperative nutrition support of the patients with pancreatic head cancer]. Acta Acad Med Sin. 2005; 27:579–582. Chinese.13. Nagata S, Fukuzawa K, Iwashita Y, Kabashima A, Kinoshita T, Wakasugi K, et al. Comparison of enteral nutrition with combined enteral and parenteral nutrition in post-pancreaticoduodenectomy patients: a pilot study. Nutr J. 2009; 8:24.
Article14. Siddique A, Kowdley KV. Approach to a patient with elevated serum alkaline phosphatase. Clin Liver Dis. 2012; 16:199–229.
Article15. Gerritsen A, Besselink MG, Cieslak KP, Vriens MR, Steenhagen E, van Hillegersberg R, et al. Efficacy and complications of nasojejunal, jejunostomy and parenteral feeding after pancreaticoduodenectomy. J Gastrointest Surg. 2012; 16:1144–1151.
Article16. Elferink MG, Olinga P, Draaisma AL, Merema MT, Faber KN, Slooff MJ, et al. LPS-induced downregulation of MRP2 and BSEP in human liver is due to a posttranscriptional process. Am J Physiol Gastrointest Liver Physiol. 2004; 287:G1008–G1016.
Article17. Green RM, Beier D, Gollan JL. Regulation of hepatocyte bile salt transporters by endotoxin and inflammatory cytokines in rodents. Gastroenterology. 1996; 111:193–198.
Article18. Geier A, Dietrich CG, Voigt S, Kim SK, Gerloff T, Kullak-Ublick GA, et al. Effects of proinflammatory cytokines on rat organic anion transporters during toxic liver injury and cholestasis. Hepatology. 2003; 38:345–354.
Article19. Cherrington NJ, Slitt AL, Li N, Klaassen CD. Lipopolysaccharide-mediated regulation of hepatic transporter mRNA levels in rats. Drug Metab Dispos. 2004; 32:734–741.
Article20. Denson LA, Auld KL, Schiek DS, McClure MH, Mangelsdorf DJ, Karpen SJ. Interleukin-1beta suppresses retinoid transactivation of two hepatic transporter genes involved in bile formation. J Biol Chem. 2000; 275:8835–8843.21. Kubitz R, Wettstein M, Warskulat U, Häussinger D. Regulation of the multidrug resistance protein 2 in the rat liver by lipopolysaccharide and dexamethasone. Gastroenterology. 1999; 116:401–410.
Article22. Hwang SE, Jung MJ, Cho BH, Yu HC. Clinical feasibility and nutritional effects of early oral feeding after pancreaticoduodenectomy. Korean J Hepatobiliary Pancreat Surg. 2014; 18:84–89.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Selection of the Enterostomy Feeding Route in Enteral Nutrition
- Enteral Feeding for Preterm Infants-Benefits and Risks
- Postoperative Nutritional Effects of Early Enteral Feeding Compared with Total Parental Nutrition in Pancreaticoduodectomy Patients: A Prosepective, Randomized Study
- Selection of Enteral Feeding in Poststroke Dysphagia
- Sources and Formulation of Macro- and Micro-Nutrients in Enteral Nutrition Formula