J Breast Cancer.
2017 Sep;20(3):297-303. 10.4048/jbc.2017.20.3.297.
Comparison of Core Needle Biopsy and Surgical Specimens in Determining Intrinsic Biological Subtypes of Breast Cancer with Immunohistochemistry
- Affiliations
-
- 1Division of Breast Surgery, Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. paojlus@hanmail.net
- 2Department of Surgery, Chungbuk National University Hospital, Chungbuk National University College of Medicine, Cheongju, Korea.
- KMID: 2438997
- DOI: http://doi.org/10.4048/jbc.2017.20.3.297
Abstract
- PURPOSE
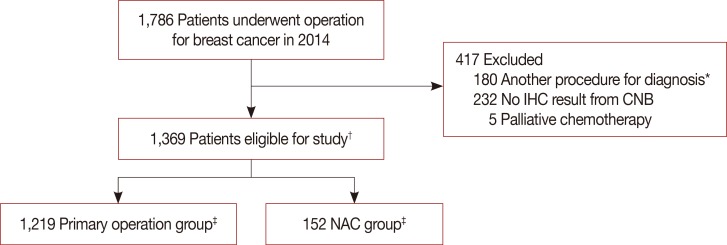
We evaluated the concordance between core needle biopsy (CNB) and surgical specimens on examining intrinsic biological subtypes and receptor status, and determined the accuracy of CNB as a basic diagnostic method.
METHODS
We analyzed breast cancer patients with paired CNB and surgical specimen samples during 2014. We used monoclonal antibodies for nuclear staining, and estrogen receptor (ER) and progesterone receptor (PR) status evaluation. A positive test was defined as staining greater than or equal to 1% of tumor cells. Human epidermal growth factor receptor 2 (HER2) was graded by immunohistochemistry and scored as 0 to 3+ according to the recommendations of the American Society of Clinical Oncology/College of American Pathologists. Ki-67 immunostaining was performed using the monoclonal antibody Ki-67, and the results were divided at 10% intervals. The cutoff value for high Ki-67 was defined as 20%. Concordance analysis of ER, PR, HER2, Ki-67, and five intrinsic biological subtypes was performed on CNB and surgical specimens. Statistical analysis for concordance was calculated using κ-tests.
RESULTS
We found very good agreement for ER and PR with a concordance of 96.7% for ER (κ=0.903), and 94.3% for PR (κ=0.870). HER2 and Ki-67 showed concordance rates of 84.8% (κ=0.684) and 83.5% (κ=0.647), respectively, which were interpreted as good agreement. Five subgroups analysis showed 85.8% agreement and κ-value of 0.786, also indicating good agreement.
CONCLUSION
CNB showed high diagnostic accuracy compared with surgical specimens, and good agreement for ER, PR, HER2, and Ki-67. Our findings reaffirmed the recommendation of CNB as an initial procedure for breast cancer diagnosis, and the assessment of receptor status and intrinsic biological subtypes to determine further treatment plans.
Keyword
MeSH Terms
-
Antibodies, Monoclonal
Biopsy, Large-Core Needle*
Breast Neoplasms*
Breast*
Diagnosis
Estrogens
Humans
Immunohistochemistry*
Methods
Receptor, Epidermal Growth Factor
Receptors, Estrogen
Receptors, Progesterone
Antibodies, Monoclonal
Estrogens
Receptor, Epidermal Growth Factor
Receptors, Estrogen
Receptors, Progesterone
Figure
Cited by 2 articles
-
Identifying breast cancer patients who require a double-check of preoperative core needle biopsy and postoperative surgical specimens to determine the molecular subtype of their tumor
Je Hyung Park, Hyun Yul Kim, Youn Joo Jung, Dong Il Kim, Jee Yeon Kim, Hyun-June Paik
Ann Surg Treat Res. 2019;97(5):223-229. doi: 10.4174/astr.2019.97.5.223.Analysis of the molecular subtypes of preoperative core needle biopsy and surgical specimens in invasive breast cancer
Ye Sul Jeong, Jun Kang, Jieun Lee, Tae-Kyung Yoo, Sung Hun Kim, Ahwon Lee
J Pathol Transl Med. 2020;54(1):87-94. doi: 10.4132/jptm.2019.10.14.
Reference
-
1. Bruening W, Fontanarosa J, Tipton K, Treadwell JR, Launders J, Schoelles K. Systematic review: comparative effectiveness of core-needle and open surgical biopsy to diagnose breast lesions. Ann Intern Med. 2010; 152:238–246. PMID: 20008742.
Article2. Pettine S, Place R, Babu S, Williard W, Kim D, Carter P. Stereotactic breast biopsy is accurate, minimally invasive, and cost effective. Am J Surg. 1996; 171:474–476. PMID: 8651388.
Article3. Arnedos M, Nerurkar A, Osin P, A'Hern R, Smith IE, Dowsett M. Discordance between core needle biopsy (CNB) and excisional biopsy (EB) for estrogen receptor (ER), progesterone receptor (PgR) and HER2 status in early breast cancer (EBC). Ann Oncol. 2009; 20:1948–1952. PMID: 19570962.
Article4. Lorgis V, Algros MP, Villanueva C, Chaigneau L, Thierry-Vuillemin A, Nguyen T, et al. Discordance in early breast cancer for tumour grade, estrogen receptor, progesteron receptors and human epidermal receptor-2 status between core needle biopsy and surgical excisional primary tumour. Breast. 2011; 20:284–287. PMID: 21288720.
Article5. Al Sarakbi W, Salhab M, Thomas V, Mokbel K. Is preoperative core biopsy accurate in determining the hormone receptor status in women with invasive breast cancer? Int Semin Surg Oncol. 2005; 2:15. PMID: 16115314.
Article6. Tamaki K, Sasano H, Ishida T, Miyashita M, Takeda M, Amari M, et al. Comparison of core needle biopsy (CNB) and surgical specimens for accurate preoperative evaluation of ER, PgR and HER2 status of breast cancer patients. Cancer Sci. 2010; 101:2074–2079. PMID: 20557310.
Article7. Ough M, Velasco J, Hieken TJ. A comparative analysis of core needle biopsy and final excision for breast cancer: histology and marker expression. Am J Surg. 2011; 201:692–694. PMID: 20850706.
Article8. Chen X, Yuan Y, Gu Z, Shen K. Accuracy of estrogen receptor, progesterone receptor, and HER2 status between core needle and open excision biopsy in breast cancer: a meta-analysis. Breast Cancer Res Treat. 2012; 134:957–967. PMID: 22370627.
Article9. Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015; 26(Suppl 5):v8–v30. PMID: 26314782.
Article10. Prowell TM, Pazdur R. Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med. 2012; 366:2438–2441. PMID: 22646508.
Article11. Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/ College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013; 31:3997–4013. PMID: 24101045.12. Dowsett M, Bartlett J, Ellis IO, Salter J, Hills M, Mallon E, et al. Correlation between immunohistochemistry (HercepTest) and fluorescence in situ hybridization (FISH) for HER-2 in 426 breast carcinomas from 37 centres. J Pathol. 2003; 199:418–423. PMID: 12635131.13. Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984; 133:1710–1715. PMID: 6206131.14. Dowsett M, Nielsen TO, A'Hern R, Bartlett J, Coombes RC, Cuzick J, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011; 103:1656–1664. PMID: 21960707.
Article15. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, et al. Strategies for subtypes: dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011; 22:1736–1747. PMID: 21709140.16. Mann GB, Fahey VD, Feleppa F, Buchanan MR. Reliance on hormone receptor assays of surgical specimens may compromise outcome in patients with breast cancer. J Clin Oncol. 2005; 23:5148–5154. PMID: 16051956.
Article17. Arens N, Bleyl U, Hildenbrand R. HER2/neu, p53, Ki67, and hormone receptors do not change during neoadjuvant chemotherapy in breast cancer. Virchows Arch. 2005; 446:489–496. PMID: 15838646.
Article18. Quddus RM, Sung JC, Zhang C, Pasqueriello T, Eklund M, Steinhoff MM. HER-2/neu expression in locally advanced breast carcinomas: pre-and post-neoadjuvant chemotherapy. Breast Cancer. 2005; 12:294–298. PMID: 16286910.19. Burge CN, Chang HR, Apple SK. Do the histologic features and results of breast cancer biomarker studies differ between core biopsy and surgical excision specimens? Breast. 2006; 15:167–172. PMID: 16095904.
Article20. Chen X, Sun L, Mao Y, Zhu S, Wu J, Huang O, et al. Preoperative core needle biopsy is accurate in determining molecular subtypes in invasive breast cancer. BMC Cancer. 2013; 13:390. PMID: 23957561.
Article21. Dekker TJ, Smit VT, Hooijer GK, Van de Vijver MJ, Mesker WE, Tollenaar RA, et al. Reliability of core needle biopsy for determining ER and HER2 status in breast cancer. Ann Oncol. 2013; 24:931–937. PMID: 23211940.
Article22. Seferina SC, Nap M, van den Berkmortel F, Wals J, Voogd AC, Tjan-Heijnen VC. Reliability of receptor assessment on core needle biopsy in breast cancer patients. Tumour Biol. 2013; 34:987–994. PMID: 23269610.
Article23. Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998; 11:155–168. PMID: 9504686.24. Zidan A, Christie Brown JS, Peston D, Shousha S. Oestrogen and progesterone receptor assessment in core biopsy specimens of breast carcinoma. J Clin Pathol. 1997; 50:27–29. PMID: 9059351.
Article25. Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010; 11:174–183. PMID: 20152769.
Article26. von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012; 30:1796–1804. PMID: 22508812.
Article27. Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014; 384:164–172. PMID: 24529560.
Article28. Bonnefoi H, Litière S, Piccart M, MacGrogan G, Fumoleau P, Brain E, et al. Pathological complete response after neoadjuvant chemotherapy is an independent predictive factor irrespective of simplified breast cancer intrinsic subtypes: a landmark and two-step approach analyses from the EORTC 10994/BIG 1-00 phase III trial. Ann Oncol. 2014; 25:1128–1136. PMID: 24618153.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinicopathologic Features of the Papillary Breast Lesions Diagnosed on Ultrasonography-guided Core Needle Biopsy
- Pseudoaneurysm of the Breast after Core Needle Biopsy: A Case Report
- Analysis of the molecular subtypes of preoperative core needle biopsy and surgical specimens in invasive breast cancer
- Track Seeding in a Breast Cancer Patient after a 14-Gauge Core Needle Biopsy: A Case Report
- Factors in the Breast Core Needle Biopsies of Atypical Ductal Hyperplasia that Can Predict Carcinoma in the Subsequent Surgical Excision Specimens