J Breast Cancer.
2017 Sep;20(3):234-239. 10.4048/jbc.2017.20.3.234.
Crotonis Fructus Extract Inhibits 12-O-Tetradecanoylphorbol-13-Acetate-Induced Expression of Matrix Metalloproteinase-9 via the Activator Protein-1 Pathway in MCF-7 Cells
- Affiliations
-
- 1Center for Metabolic Function Regulation, Wonkwang University School of Medicine, Iksan, Korea. desson@wku.ac.kr
- 2Department of Herbology, Wonkwang University School of Korean Medicine, Iksan, Korea.
- 3Department of Korean Physiology, Wonkwang University School of Korean Medicine, Iksan, Korea.
- 4Department of Surgery, Biomedical Research Institute of Chonbuk National University Hospital, Research Institute of Clinical Medicine of Chonbuk National University, Jeonju, Korea.
- 5Department of Oral Biochemistry and Institute of Biomaterials-Implant, Wonkwang University School of Dentistry, Iksan, Korea.
- 6Integrated Omics Institute, Wonkwang University, Iksan, Korea.
- KMID: 2438989
- DOI: http://doi.org/10.4048/jbc.2017.20.3.234
Abstract
- PURPOSE
Metastatic cancers spread from the primary site of origin to other parts of the body. Matrix metalloproteinase-9 (MMP-9) is essential in metastatic cancers owing to its major role in cancer cell invasion. Crotonis fructus (CF), the mature fruits of Croton tiglium L., have been used for the treatment of gastrointestinal disturbance in Asia. In this study, the effect of the ethanol extract of CF (CFE) on MMP-9 activity and the invasion of 12-O-tetradecanoylphorbol-13-acetate (TPA)-treated MCF-7 cells was examined.
METHODS
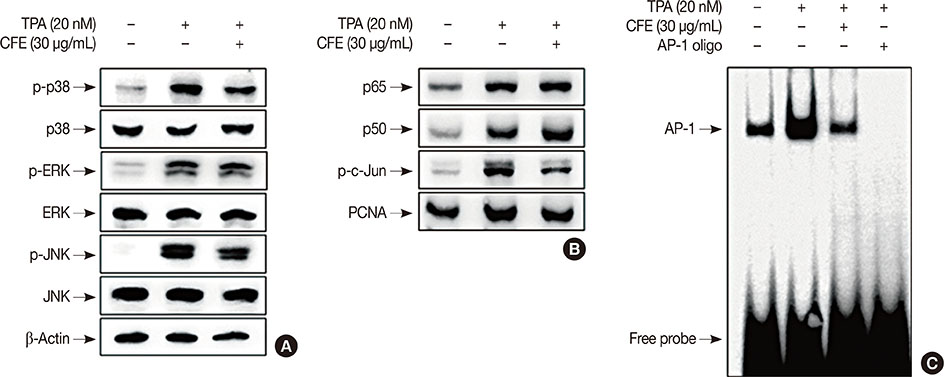
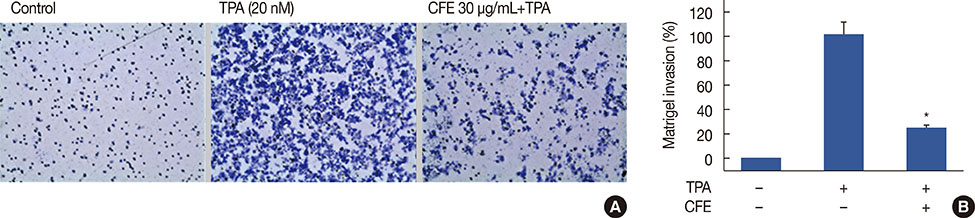
The cell viability was evaluated using the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. The expression of MMP-9 was examined by Western blotting, zymography, and real-time polymerase chain reaction. An electrophoretic mobility gel shift assay was performed to detect activator protein-1 (AP-1) DNA binding activity and cell invasiveness was measured by an in vitro Matrigel invasion assay.
RESULTS
CFE significantly suppressed MMP-9 expression and activation in a dose-dependent manner. Furthermore, CFE attenuated the TPA-induced activation of AP-1.
CONCLUSION
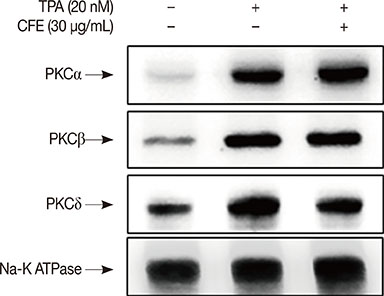
The results indicated that the inhibitory effects of CFE against TPA-induced MMP-9 expression and MCF-7 cell invasion were dependent on the protein kinase C δ/p38/c-Jun N-terminal kinase/AP-1 pathway. Therefore, CFE could restrict breast cancer invasiveness owing to its ability to inhibit MMP-9 activity.
Keyword
MeSH Terms
-
Asia
Blotting, Western
Breast Neoplasms
Cell Survival
Croton
DNA
Ethanol
Fruit
In Vitro Techniques
Matrix Metalloproteinase 9*
MCF-7 Cells*
Neoplasm Invasiveness
Protein Kinase C
Real-Time Polymerase Chain Reaction
Transcription Factor AP-1*
DNA
Ethanol
Matrix Metalloproteinase 9
Protein Kinase C
Transcription Factor AP-1
Figure
Reference
-
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–E386.
Article2. Yücel B, Bahar S, Kaçan T, Şeker M, Celasun MG, Bahçeci A, et al. Importance of metastasis site in survival of patients with breast cancer. Austin J Med Oncol. 2014; 1:7.3. Eckhardt BL, Francis PA, Parker BS, Anderson RL. Strategies for the discovery and development of therapies for metastatic breast cancer. Nat Rev Drug Discov. 2012; 11:479–497.
Article4. Woessner JF Jr. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991; 5:2145–2154.
Article5. Park HI, Ni J, Gerkema FE, Liu D, Belozerov VE, Sang QX. Identification and characterization of human endometase (matrix metalloproteinase-26) from endometrial tumor. J Biol Chem. 2000; 275:20540–20544.
Article6. Mylona E, Nomikos A, Magkou C, Kamberou M, Papassideri I, Keramopoulos A, et al. The clinicopathological and prognostic significance of membrane type 1 matrix metalloproteinase (MT1-MMP) and MMP-9 according to their localization in invasive breast carcinoma. Histopathology. 2007; 50:338–347.
Article7. Tsai JC, Tsai S, Chang WC. Effect of ethanol extracts of three Chinese medicinal plants with anti-diarrheal properties on ion transport of the rat intestinal epithelia. J Pharmacol Sci. 2004; 94:60–66.
Article8. Wang X, Lan M, Wu HP, Shi YQ, Lu J, Ding J, et al. Direct effect of croton oil on intestinal epithelial cells and colonic smooth muscle cells. World J Gastroenterol. 2002; 8:103–107.
Article9. Kim JH, Lee SJ, Han YB, Moon JJ, Kim JB. Isolation of isoguanosine from Croton tiglium and its antitumor activity. Arch Pharm Res. 1994; 17:115–118.
Article10. Eberhardt W, Huwiler A, Beck KF, Walpen S, Pfeilschifter J. Amplification of IL-1 beta-induced matrix metalloproteinase-9 expression by superoxide in rat glomerular mesangial cells is mediated by increased activities of NF-kappa B and activating protein-1 and involves activation of the mitogen-activated protein kinase pathways. J Immunol. 2000; 165:5788–5797.
Article11. Lungu G, Covaleda L, Mendes O, Martini-Stoica H, Stoica G. FGF-1-induced matrix metalloproteinase-9 expression in breast cancer cells is mediated by increased activities of NF-kappaB and activating protein-1. Mol Carcinog. 2008; 47:424–435.
Article12. Nabeshima K, Inoue T, Shimao Y, Sameshima T. Matrix metalloproteinases in tumor invasion: role for cell migration. Pathol Int. 2002; 52:255–264.
Article13. Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997; 89:1260–1270.
Article14. Hetter GP. An examination of the phenol-croton oil peel: part I. dissecting the formula. Plast Reconstr Surg. 2000; 105:227–239.
Article15. Jeon CM, Min GW, Park JH, Yoon CH, Jeong JC, Kang JJ, et al. Effect of the methanol extract of Dansameum on the ammonia-induced peptic ulcer in rats. Korean J Orient Int Med. 2000; 21:597–604.16. Kim MS, Kim HR, So HS, Lee YR, Moon HC, Ryu DG, et al. Crotonis fructus and its constituent, croton oil, stimulate lipolysis in OP9 adipocytes. Evid Based Complement Alternat Med. 2014; 2014:780385.17. Kim KC, Lee CH. MAP kinase activation is required for the MMP-9 induction by TNF-stimulation. Arch Pharm Res. 2005; 28:1257–1262.
Article18. Hong S, Park KK, Magae J, Ando K, Lee TS, Kwon TK, et al. Ascochlorin inhibits matrix metalloproteinase-9 expression by suppressing activator protein-1-mediated gene expression through the ERK1/2 signaling pathway: inhibitory effects of ascochlorin on the invasion of renal carcinoma cells. J Biol Chem. 2005; 280:25202–25209.
Article19. Karin M, Liu Zg, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997; 9:240–246.
Article20. Crawford HC, Matrisian LM. Mechanisms controlling the transcription of matrix metalloproteinase genes in normal and neoplastic cells. Enzyme Protein. 1996; 49:20–37.
Article21. Kim JM, Noh EM, Kwon KB, Kim JS, You YO, Hwang JK, et al. Curcumin suppresses the TPA-induced invasion through inhibition of PKC-alpha-dependent MMP-expression in MCF-7 human breast cancer cells. Phytomedicine. 2012; 19:1085–1092.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Doxycycline on PMA-Induced MUC5B Expression via MMP-9 and p38 in NCI-H292 Cells
- Troglitazone Inhibits Matrix Metalloproteinase-9 Expression and Invasion of Breast Cancer Cell through a Peroxisome Proliferator-Activated Receptor γ-Dependent Mechanism
- 12-O-Tetradecanoylphorbol-13-Acetate Induces Keratin 8 Phosphorylation and Reorganization via Expression of Transglutaminase-2
- Effect of Epigallocatechin-3-Gallate on PMA-Induced MUC5B Expression in Human Airway Epithelial Cells
- PMA-induced up-regulation of MMP-9 is regulated by a PKCalpha-NF-kappaB cascade in human lung epithelial cells