Investig Clin Urol.
2019 Mar;60(2):91-98. 10.4111/icu.2019.60.2.91.
Characterization of human infiltrating and circulating gamma-delta T cells in prostate cancer
- Affiliations
-
- 1Department of Surgical Oncological and Oral Sciences, Section of Urology, University of Palermo, Palermo, Italy. marco.vella@unipa.it
- 2Department of Biopathology and Clinical and Forensic Biotechnology, University of Palermo, Palermo, Italy.
- KMID: 2438819
- DOI: http://doi.org/10.4111/icu.2019.60.2.91
Abstract
- PURPOSE
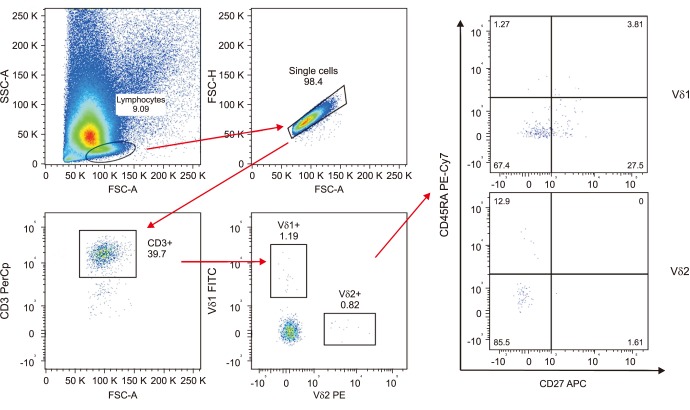
The aim of our study was to prospectively evaluate the distribution of gamma-delta (γδ)1 and γδ2 T cells and their phenotypes in peripheral blood and prostate samples of patients diagnosed with or without prostate cancer (PCa) at prostate biopsy.
MATERIALS AND METHODS
A consecutive series of 43 outpatients underwent trans-rectal echo-guided prostate biopsy for suspected PCa. Flow cytometry analysis was used to identify and characterize the γδ T cells populations in peripheral blood and tissue samples. Patients were stratified according to the presence or not of PCa, and its International Society of Urological Pathology (ISUP) grade (1 vs. ≥2).
RESULTS
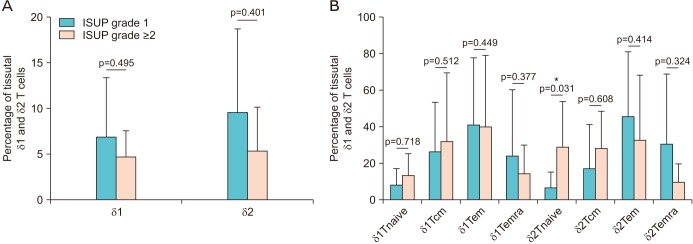
The distribution of γδ T cells in peripheral blood and prostate tissue showed wide variability and non-significant differences. A slightly higher percentage of δ2 T cells and a slightly lower percentage of δ1 T cells were found in peripheral blood of cancer patients. A non-significantly higher percentage of both Vδ1 and Vδ2 was expressed in cancer tissues, but a trend for lower distribution of δ1 and δ2 T cells was observed in ISUP grade ≥2. The "central memory" and "effector memory" were the most expressed T cells phenotype in peripheral blood and tissue samples. However no substantial differences in T cells subtypes distribution between cancer and healthy tissue were observed.
CONCLUSIONS
No substantially different percentages of γδ T cells were found in peripheral blood and biopsy samples of healthy and PCa patients. However a non-significant trend for lower infiltrate in higher ISUP grade cancer tissue was observed, suggesting a possible role for the immunosurveillance of PCa.
Keyword
MeSH Terms
Figure
Reference
-
1. Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005; 293:2095–2101. PMID: 15870412.
Article2. Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003; 349:215–224. PMID: 12824459.
Article3. De Marzo AM, Platz EA, Sutcliffe S, Xu J, Grönberg H, Drake CG, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007; 7:256–269. PMID: 17384581.
Article4. Hao J, Wu X, Xia S, Li Z, Wen T, Zhao N, et al. Current progress in γδ T-cell biology. Cell Mol Immunol. 2010; 7:409–413. PMID: 21042298.
Article5. Dieli F, Gebbia N, Poccia F, Caccamo N, Montesano C, Fulfaro F, et al. Induction of gammadelta T-lymphocyte effector functions by bisphosphonate zoledronic acid in cancer patients in vivo. Blood. 2003; 102:2310–2311. PMID: 12959943.6. Moser B, Eberl M. γδ T-APCs: a novel tool for immunotherapy? Cell Mol Life Sci. 2011; 68:2443–2452. PMID: 21573785.
Article7. Tyler CJ, McCarthy NE, Lindsay JO, Stagg AJ, Moser B, Eberl M. Antigen-presenting human γδ T cells promote intestinal CD4+ T cell expression of IL-22 and mucosal release of calprotectin. J Immunol. 2017; 198:3417–3425. PMID: 28330898.8. Mangan BA, Dunne MR, O'Reilly VP, Dunne PJ, Exley MA, O'Shea D, et al. Cutting edge: CD1d restriction and Th1/Th2/Th17 cytokine secretion by human Vδ3 T cells. J Immunol. 2013; 191:30–34. PMID: 23740951.
Article9. Rei M, Pennington DJ, Silva-Santos B. The emerging protumor role of γδ T lymphocytes: implications for cancer immunotherapy. Cancer Res. 2015; 75:798–802. PMID: 25660949.
Article10. Urban EM, Chapoval AI, Pauza CD. Repertoire development and the control of cytotoxic/effector function in human gammadelta T cells. Clin Dev Immunol. 2010; 2010:732893. PMID: 20396597.11. Dieli F, Poccia F, Lipp M, Sireci G, Caccamo N, Di Sano C, et al. Differentiation of effector/memory Vdelta2 T cells and migratory routes in lymph nodes or inflammatory sites. J Exp Med. 2003; 198:391–397. PMID: 12900516.12. Jensen KD, Chien YH. Thymic maturation determines gammadelta T cell function, but not their antigen specificities. Curr Opin Immunol. 2009; 21:140–145. PMID: 19321327.13. Tortorici S, Argo A, Buzzanca ML, Burruano F, Tetè S. Information, consent and therapeutic alliance in ambulatorial oral surgery. Ital Oral Surg. 2009; 8:155–164.14. Rei M, Gonçalves-Sousa N, Lança T, Thompson RG, Mensurado S, Balkwill FR, et al. Murine CD27(−) Vγ6(+) γδ T cells producing IL-17A promote ovarian cancer growth via mobilization of protumor small peritoneal macrophages. Proc Natl Acad Sci U S A. 2014; 111:E3562–E3570. PMID: 25114209.
Article15. Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015; 21:938–945. PMID: 26193342.
Article16. Ma S, Cheng Q, Cai Y, Gong H, Wu Y, Yu X, et al. IL-17A produced by γδ T cells promotes tumor growth in hepatocellular carcinoma. Cancer Res. 2014; 74:1969–1982. PMID: 24525743.
Article17. Inman BA, Frigola X, Harris KJ, Kuntz SM, Lohse CM, Leibovich BC, et al. Questionable relevance of gamma delta T lymphocytes in renal cell carcinoma. J Immunol. 2008; 180:3578–3584. PMID: 18292585.18. Ma C, Zhang Q, Ye J, Wang F, Zhang Y, Wevers E, et al. Tumor-infiltrating γδ T lymphocytes predict clinical outcome in human breast cancer. J Immunol. 2012; 189:5029–5036. PMID: 23034170.
Article19. Rhodes KA, Andrew EM, Newton DJ, Tramonti D, Carding SR. A subset of IL-10-producing gammadelta T cells protect the liver from Listeria-elicited, CD8(+) T cell-mediated injury. Eur J Immunol. 2008; 38:2274–2283. PMID: 18624301.20. Jensen KD, Su X, Shin S, Li L, Youssef S, Yamasaki S, et al. Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008; 29:90–100. PMID: 18585064.21. Wu X, Zhang JY, Huang A, Li YY, Zhang S, Wei J, et al. Decreased Vδ2 γδ T cells associated with liver damage by regulation of Th17 response in patients with chronic hepatitis B. J Infect Dis. 2013; 208:1294–1304. PMID: 23847059.
Article22. Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruediger T, et al. Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood. 2003; 102:200–206. PMID: 12623838.23. Kobayashi H, Tanaka Y, Yagi J, Osaka Y, Nakazawa H, Uchiyama T, et al. Safety profile and anti-tumor effects of adoptive immunotherapy using gamma-delta T cells against advanced renal cell carcinoma: a pilot study. Cancer Immunol Immunother. 2007; 56:469–476. PMID: 16850345.
Article24. Guo RT, Cao R, Liang PH, Ko TP, Chang TH, Hudock MP, et al. Bisphosphonates target multiple sites in both cis- and transprenyltransferases. Proc Natl Acad Sci U S A. 2007; 104:10022–10027. PMID: 17535895.
Article25. Ebelt K, Babaryka G, Frankenberger B, Stief CG, Eisenmenger W, Kirchner T, et al. Prostate cancer lesions are surrounded by FOXP3+, PD-1+ and B7-H1+ lymphocyte clusters. Eur J Cancer. 2009; 45:1664–1672. PMID: 19318244.
Article26. Sfanos KS, Bruno TC, Meeker AK, De Marzo AM, Isaacs WB, Drake CG. Human prostate-infiltrating CD8+ T lymphocytes are oligoclonal and PD-1+. Prostate. 2009; 69:1694–1703. PMID: 19670224.
Article27. Miller AM, Lundberg K, Ozenci V, Banham AH, Hellström M, Egevad L, et al. CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol. 2006; 177:7398–7405. PMID: 17082659.
Article28. Norström MM, Rådestad E, Sundberg B, Mattsson J, Henningsohn L, Levitsky V, et al. Progression of benign prostatic hyperplasia is associated with pro-inflammatory mediators and chronic activation of prostate-infiltrating lymphocytes. Oncotarget. 2016; 7:23581–23593. PMID: 26993768.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gamma/delta T lymphocytes in the BCG granulomatous lesions
- Translational Application of Single-cell Transcriptomic Analysis in Hepatocellular Carcinoma
- Identification of Enhancer of Zeste Homolog 2 Expression in Peripheral Circulating Tumor Cells in Metastatic Prostate Cancer Patients: A Preliminary Study
- Changes of Natural Killer T Cells in Behcet's Uveitis
- Molecular characterization of the feline T-cell receptor gamma alternate reading frame protein (TARP) ortholog