Intest Res.
2019 Jan;17(1):87-93. 10.5217/ir.2018.00078.
Randomized, crossover questionnaire survey of acceptabilities of controlled-release mesalazine tablets and granules in ulcerative colitis patients
- Affiliations
-
- 1Department of Pharmacy, Kitasato University Kitasato Institute Hospital, Tokyo, Japan.
- 2Center for Advanced IBD Research and Treatment, Kitasato University Kitasato Institute Hospital, Tokyo, Japan. kobataku@insti.kitasato-u.ac.jp
- 3The Third Department of Internal Medicine, Kyorin University School of Medicine, Tokyo, Japan.
- KMID: 2438447
- DOI: http://doi.org/10.5217/ir.2018.00078
Abstract
- BACKGROUND/AIMS
Oral mesalazine is an important treatment for ulcerative colitis (UC), and non-adherence to mesalazine increases the risk of relapse. Controlled-release (CR) mesalazine has 2 formulations: tablets and granules. The relative acceptabilities of these formulations may influence patient adherence; however, they have not been compared to date. This study aimed to evaluate the acceptabilities of the 2 formulations of CR mesalazine in relation to patient adherence using a crossover questionnaire survey.
METHODS
UC patients were randomly assigned to 2 groups in a 1:1 ratio. Patients in each group took either 4 g of CR mesalazine tablets or granules for 6 to 9 weeks, and then switched to 4 g of the other formulation for a further 6 to 9 weeks. The acceptability and efficacy were evaluated by questionnaires, and adherence was assessed using a visual analog scale. The difference in acceptabilities between the 2 formulations and its impact on adherence were assessed.
RESULTS
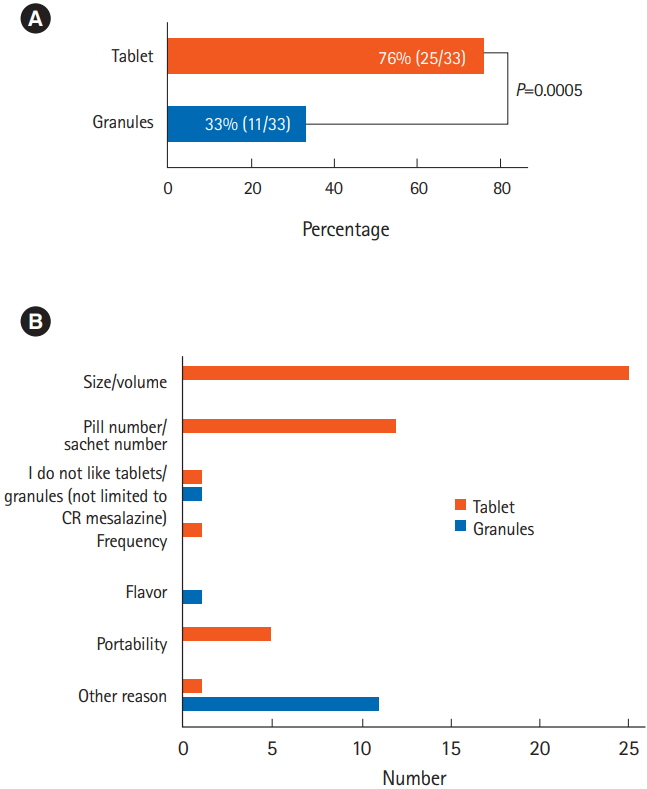
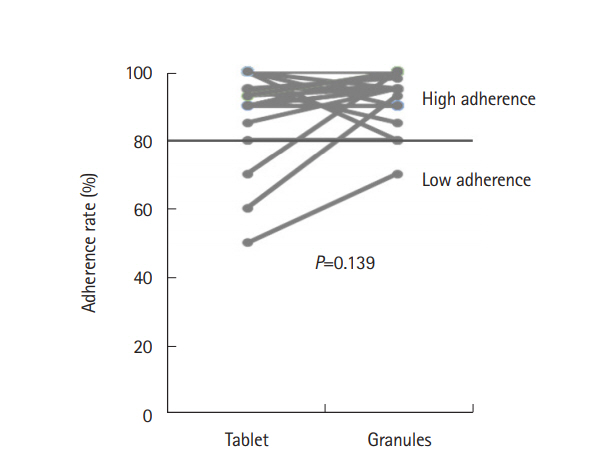
A total of 49 patients were prospectively enrolled and 33 patients were included in the analysis. Significantly more patients found the tablets to be less acceptable than the granules (76% vs. 33%, P=0.0005). The granules were preferable to the tablets when the 2 formulations were compared directly (73% vs. 21%, P=0.004), for their portability, size, and numbers of pills. The adherence rate was slightly better among patients taking the granules (94% vs. 91%) during the observation period, but the difference was not significant (P=0.139).
CONCLUSIONS
CR mesalazine granules are more acceptable than tablets, and may therefore be a better option for long-term medication.
Keyword
MeSH Terms
Figure
Reference
-
1. Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017; 11:649–670.
Article2. Kane S, Huo D, Aikens J, Hanauer S. Medication nonadherence and the outcomes of patients with quiescent ulcerative colitis. Am J Med. 2003; 114:39–43.
Article3. Kawakami A, Tanaka M, Nishigaki M, et al. Relationship between non-adherence to aminosalicylate medication and the risk of clinical relapse among Japanese patients with ulcerative colitis in clinical remission: a prospective cohort study. J Gastroenterol. 2013; 48:1006–1015.
Article4. Hanauer S, Schwartz J, Robinson M, et al. Mesalamine capsules for treatment of active ulcerative colitis: results of a controlled trial. Pentasa Study Group. Am J Gastroenterol. 1993; 88:1188–1197.5. Flourié B, Hagège H, Tucat G, et al. Randomised clinical trial: once- vs. twice-daily prolonged-release mesalazine for active ulcerative colitis. Aliment Pharmacol Ther. 2013; 37:767–775.
Article6. Wilding IR, Kenyon CJ, Hooper G. Gastrointestinal spread of oral prolonged-release mesalazine microgranules (Pentasa) dosed as either tablets or sachet. Aliment Pharmacol Ther. 2000; 14:163–169.
Article7. Devlen J, Beusterien K, Yen L, Ahmed A, Cheifetz AS, Moss AC. Barriers to mesalamine adherence in patients with inflammatory bowel disease: a qualitative analysis. J Manag Care Spec Pharm. 2014; 20:309–314.
Article8. Kawakami A, Tanaka M, Ochiai R, et al. Difficulties in taking aminosalicylates for patients with ulcerative colitis. Gastroenterol Nurs. 2012; 35:24–31.
Article9. Severs M, Zuithoff PN, Mangen MJ, et al. Assessing self-reported medication adherence in inflammatory bowel disease: a comparison of tools. Inflamm Bowel Dis. 2016; 22:2158–2164.
Article10. Ford AC, Khan KJ, Sandborn WJ, Kane SV, Moayyedi P. Oncedaily dosing vs. conventional dosing schedule of mesalamine and relapse of quiescent ulcerative colitis: systematic review and meta-analysis. Am J Gastroenterol. 2011; 106:2070–2077.
Article11. McLaughlin T, Hogue SL, Stang PE. Once-daily bupropion associated with improved patient adherence compared with twice-daily bupropion in treatment of depression. Am J Ther. 2007; 14:221–225.
Article12. Amara W, Antoniou S. Benefits of once-daily dosing with nonvitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J Suppl. 2016; 18(Suppl D):D1–D6.
Article13. National Collaborating Centre for Primary Care (UK). Medicines adherence: involving patients in decisions about prescribed medicines and supporting adherence. London: Royal College of General Practitioners (UK);2009.14. Nachega JB, Parienti JJ, Uthman OA, et al. Lower pill burden and once-daily antiretroviral treatment regimens for HIV infection: a meta-analysis of randomized controlled trials. Clin Infect Dis. 2014; 58:1297–1307.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Corrigendum: Randomized, crossover questionnaire survey of acceptabilities of controlled-release mesalazine tablets and granules in ulcerative colitis patients
- Comparison of efficacies of once-daily dose multimatrix mesalazine and multiple-dose mesalazine for the maintenance of remission in ulcerative colitis: a randomized, double-blind study
- Comparison of efficacy of once daily multimatrix mesalazine 2.4 g/day and 4.8 g/day with other 5-aminosalicylic acid preparation in active ulcerative colitis: a randomized, double-blind study
- Comparison of efficacy of multimatrix mesalazine 4.8 g/day once-daily with other high-dose mesalazine in active ulcerative colitis: a randomized, double-blind study
- Recurrent Eosinophilic Pneumonia Associated with Mesalazine Suppository in a Patient with Ulcerative Colitis