Korean J Radiol.
2019 Mar;20(3):459-468. 10.3348/kjr.2018.0464.
Hepatic Artery Occlusion after Liver Transplantation in Patients with Doppler Ultrasound Abnormality: Increasing Sensitivity of Contrast-Enhanced Ultrasound Diagnosis
- Affiliations
-
- 1Department of Radiology and Medical Research Institute, College of Medicine, Ewha Womans University, Seoul, Korea.
- 2Department of Radiology and Research Institute of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. kimkw@amc.seoul.kr
- 3Division of Liver Transplantation and Hepatobiliary Surgery, Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2438276
- DOI: http://doi.org/10.3348/kjr.2018.0464
Abstract
OBJECTIVE
To investigate whether diagnostic performance of contrast-enhanced ultrasound (CEUS) could be improved with modified criteria to diagnose significant hepatic artery occlusion (HAO) and to determine the role of CEUS in patients with a tardus-parvus hepatic artery (HA) pattern on Doppler US.
MATERIALS AND METHODS
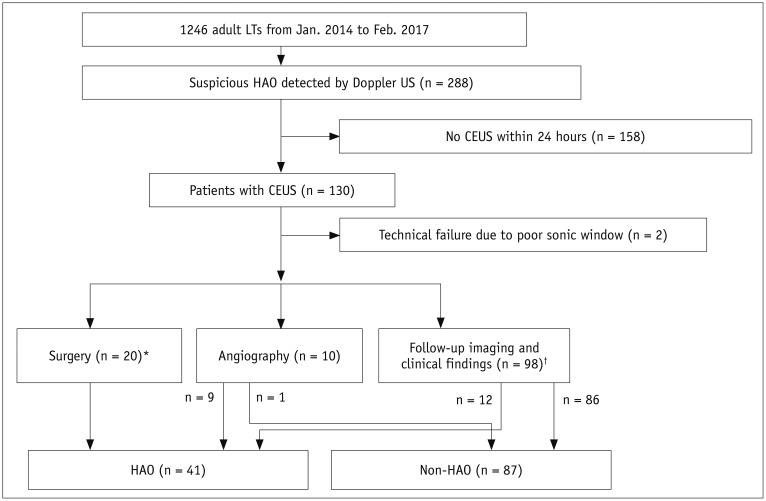
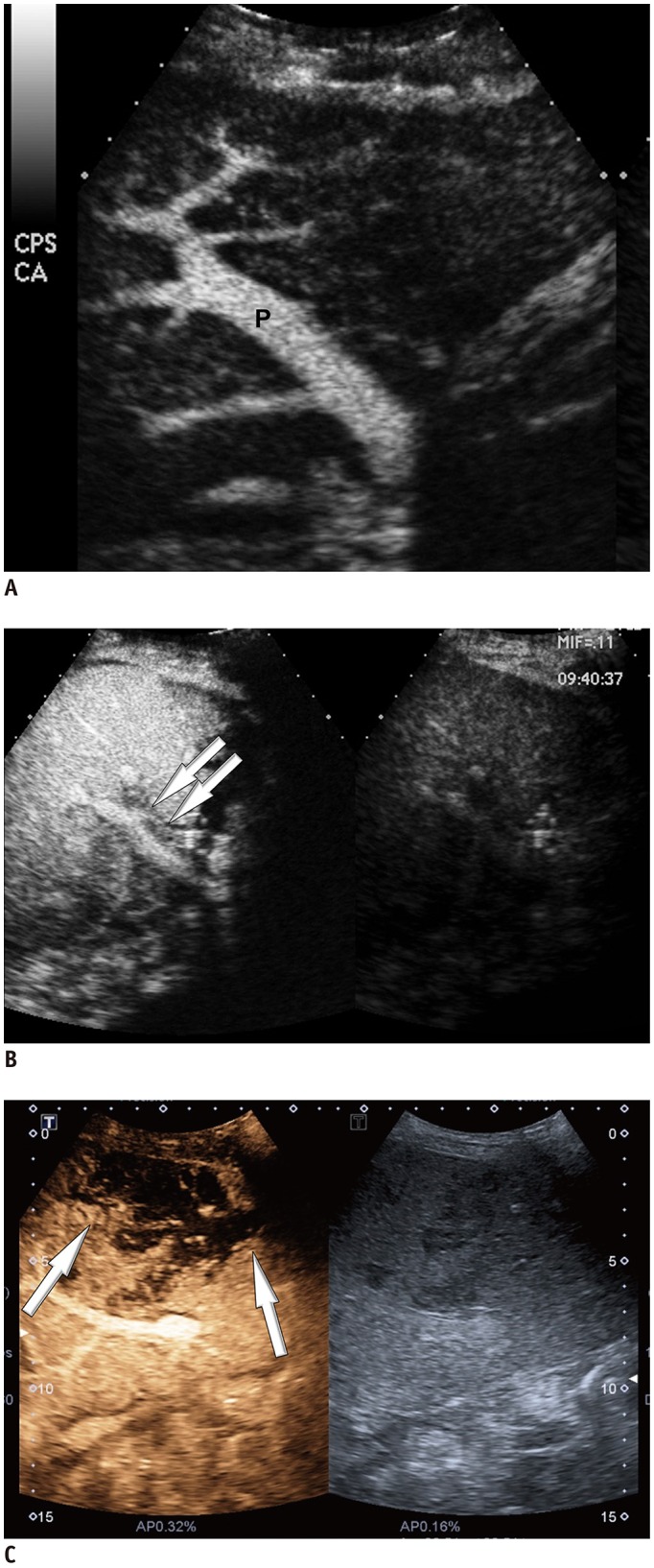
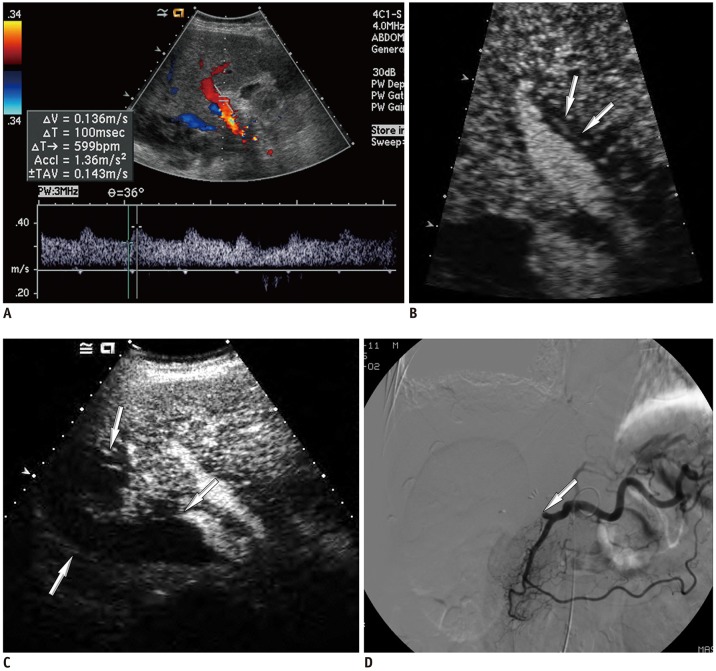
Among 2679 adult liver transplantations performed over 7 years, HAO was suspected in 288 recipients, based on Doppler ultrasound. Among them, 130 patients underwent CEUS. After excluding two technical failures, 128 CEUS images were retrospectively reviewed to search for abnormal findings, such as no HA enhancement, abnormal HA enhancement (delayed, faint, and discontinuous enhancement), and perfusion defect in the liver parenchyma. The performance CEUS abnormalities were assessed in the patients overall and in subgroups based on Doppler ultrasound abnormality (group A, no flow; group B, tardus-parvus pattern) and were compared based on the area under the receiver operating characteristic curve (AUC).
RESULTS
HAO were diagnosed in 41 patients by surgery, angiography, or follow-up abnormality. By using the conventional criterion (no HA enhancement) to diagnose HAO in patients overall, the sensitivity, specificity, and AUC were 58.5%, 100%, and 0.793, respectively. Modified criteria for HAO (no HA enhancement, abnormal enhancement, or parenchymal perfusion defect) showed statistically significantly increased sensitivity (97.6%, 40/41) and AUC (0.959) (p < 0.001), although the specificity (95.4%, 83/87) was slightly decreased. The sensitivity and specificity of the modified criteria in Groups A and B were 97.1% (33/34) and 95.7% (22/23), and 100% (7/7) and 95.3% (61/64), respectively.
CONCLUSION
Modified criteria could improve diagnostic performance of CEUS for HAO, particularly by increasing sensitivity. CEUS could be useful for diagnosing HAO even in patients with a tardus-parvus HA pattern on Doppler US, using modified criteria.
Keyword
MeSH Terms
Figure
Reference
-
1. Bekker J, Ploem S, de Jong KP. Early hepatic artery thrombosis after liver transplantation: a systematic review of the incidence, outcome and risk factors. Am J Transplant. 2009; 9:746–757. PMID: 19298450.
Article2. Gad EH, Abdelsamee MA, Kamel Y. Hepatic arterial and portal venous complications after adult and pediatric living donor liver transplantation, risk factors, management and outcome (a retrospective cohort study). Ann Med Surg (Lond). 2016; 8:28–39. PMID: 27257483.
Article3. Orons PD, Sheng R, Zajko AB. Hepatic artery stenosis in liver transplant recipients: prevalence and cholangiographic appearance of associated biliary complications. AJR Am J Roentgenol. 1995; 165:1145–1149. PMID: 7572493.
Article4. Valente JF, Alonso MH, Weber FL, Hanto DW. Late hepatic artery thrombosis in liver allograft recipients is associated with intrahepatic biliary necrosis. Transplantation. 1996; 61:61–65. PMID: 8560575.
Article5. Stange BJ, Glanemann M, Nuessler NC, Settmacher U, Steinmüller T, Neuhaus P. Hepatic artery thrombosis after adult liver transplantation. Liver Transpl. 2003; 9:612–620. PMID: 12783404.6. Pinna AD, Smith CV, Furukawa H, Starzl TE, Fung JJ. Urgent revascularization of liver allografts after early hepatic artery thrombosis. Transplantation. 1996; 62:1584–1587. PMID: 8970612.7. Singhal A, Stokes K, Sebastian A, Wright HI, Kohli V. Endovascular treatment of hepatic artery thrombosis following liver transplantation. Transpl Int. 2010; 23:245–256. PMID: 20030796.
Article8. Park YS, Kim KW, Lee SJ, Lee J, Jung DH, Song GW, et al. Hepatic arterial stenosis assessed with doppler us after liver transplantation: frequent false-positive diagnoses with tardus parvus waveform and value of adding optimal peak systolic velocity cutoff. Radiology. 2011; 260:884–891. PMID: 21734158.
Article9. Sidhu PS, Shaw AS, Ellis SM, Karani JB, Ryan SM. Microbubble ultrasound contrast in the assessment of hepatic artery patency following liver transplantation: role in reducing frequency of hepatic artery arteriography. Eur Radiol. 2004; 14:21–30. PMID: 14530998.
Article10. Zheng RQ, Mao R, Ren J, Xu EJ, Liao M, Wang P, et al. Contrast-enhanced ultrasound for the evaluation of hepatic artery stenosis after liver transplantation: potential role in changing the clinical algorithm. Liver Transpl. 2010; 16:729–735. PMID: 20517906.
Article11. Rubenthaler J, Paprottka KJ, Hameister E, Hoffmann K, Joiko N, Reiser M, et al. Diagnostic accuracy of contrast-enhanced ultrasound (CEUS) in monitoring vascular complications in patients after liver transplantation - diagnostic performance compared with histopathological results. Clin Hemorheol Microcirc. 2017; 66:311–316. PMID: 28527202.12. Hom BK, Shrestha R, Palmer SL, Katz MD, Selby RR, Asatryan Z, et al. Prospective evaluation of vascular complications after liver transplantation: comparison of conventional and microbubble contrast-enhanced US. Radiology. 2006; 241:267–274. PMID: 16990679.
Article13. Sidhu PS, Ellis SM, Karani JB, Ryan SM. Hepatic artery stenosis following liver transplantation: significance of the tardus parvus waveform and the role of microbubble contrast media in the detection of a focal stenosis. Clin Radiol. 2002; 57:789–799. PMID: 12384104.
Article14. Dodd G 3rd, Memel DS, Zajko AB, Baron RL, Santaguida LA. Hepatic artery stenosis and thrombosis in transplant recipients: doppler diagnosis with resistive index and systolic acceleration time. Radiology. 1994; 192:657–661. PMID: 8058930.
Article15. García-Criado Á, Gilabert R, Berzigotti A, Brú C. Doppler ultrasound findings in the hepatic artery shortly after liver transplantation. AJR Am J Roentgenol. 2009; 193:128–135. PMID: 19542404.
Article16. Park YS, Kim KW, Kim SY, Lee SJ, Lee J, Kim JH, et al. Obstruction at middle hepatic venous tributaries in modified right lobe grafts after living-donor liver transplantation: diagnosis with contrast-enhanced US. Radiology. 2012; 265:617–626. PMID: 22923713.
Article17. Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver - update 2012: a wfumb-efsumb initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol. 2013; 39:187–210. PMID: 23137926.18. Lyshchik A, Kono Y, Dietrich CF, Jang HJ, Kim TK, Piscaglia F, et al. Contrast-enhanced ultrasound of the liver: technical and lexicon recommendations from the ACR CEUS LI-RADS working group. Abdom Radiol (NY). 2018; 43:861–887. PMID: 29151131.
Article19. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977; 33:159–174. PMID: 843571.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of Early Vascular Complications after Liver Transplantation: Usefulness of Power Doppler US with a Microbubble Contrast Agent - Preliminary Results
- Improving the Specificity of CT Angiography for the Diagnosis of Hepatic Artery Occlusion after Liver Transplantation in Suspected Patients with Doppler Ultrasound Abnormalities
- Clinical use of contrast-enhanced ultrasound beyond the liver: a focus on renal, splenic, and pancreatic applications
- Color-flow doppler ultrasound findings of femoral artery occlusion in infants and young children
- Doppler US Findings of Vascular Complication after Liver Transplantation