Korean J Radiol.
2019 Mar;20(3):449-458. 10.3348/kjr.2018.0469.
Evaluation of Early Response to Treatment of Hepatocellular Carcinoma with Yttrium-90 Radioembolization Using Quantitative Computed Tomography Analysis
- Affiliations
-
- 1Department of Radiology, Severance Hospital, Research Institute of Radiological Science and Center for Clinical Imaging Data Science, Yonsei University College of Medicine, Seoul, Korea. gafield2@yuhs.ac
- 2Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2438275
- DOI: http://doi.org/10.3348/kjr.2018.0469
Abstract
OBJECTIVE
To identify an imaging predictor for the assessment of early treatment response to yttrium-90 transarterial radioembolization (TARE) in patients with hepatocellular carcinoma (HCC), using a quantitative assessment of dynamic computed tomography (CT) images.
MATERIALS AND METHODS
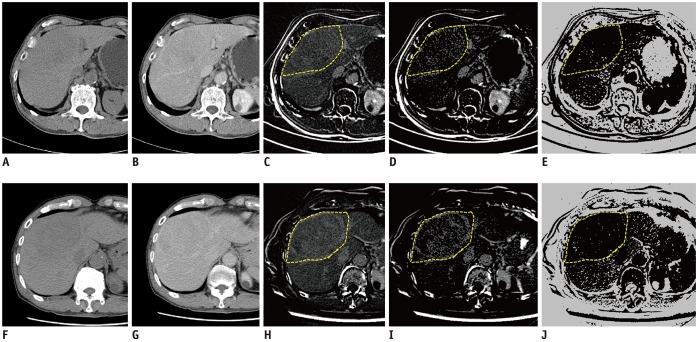
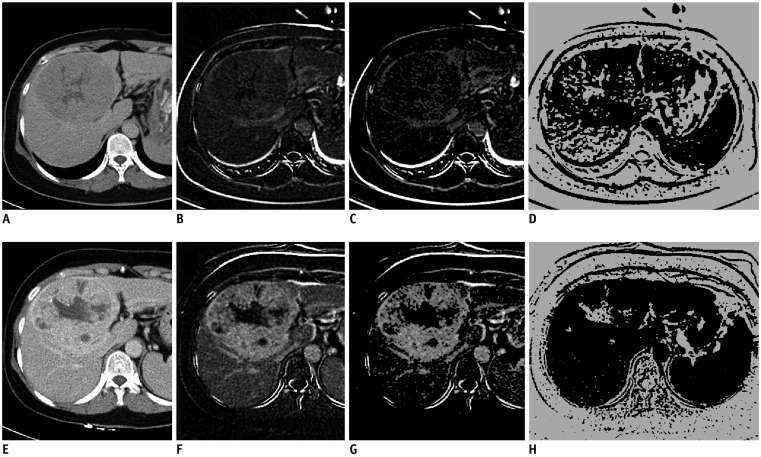
Dynamic contrast-enhanced CT was obtained pre- and 4 weeks post-TARE in 44 patients (34 men, 10 women; mean age, 60 years) with HCC. Computer software was developed for measuring the percentage increase in the combined delayed-enhancing area and necrotic area (pD + N) and the percentage increase in the necrotic area (pNI) in the tumor-containing segments pre- and post-TARE. Local progression-free survival (PFS) was compared between patient groups using Cox regression and Kaplan-Meier analyses.
RESULTS
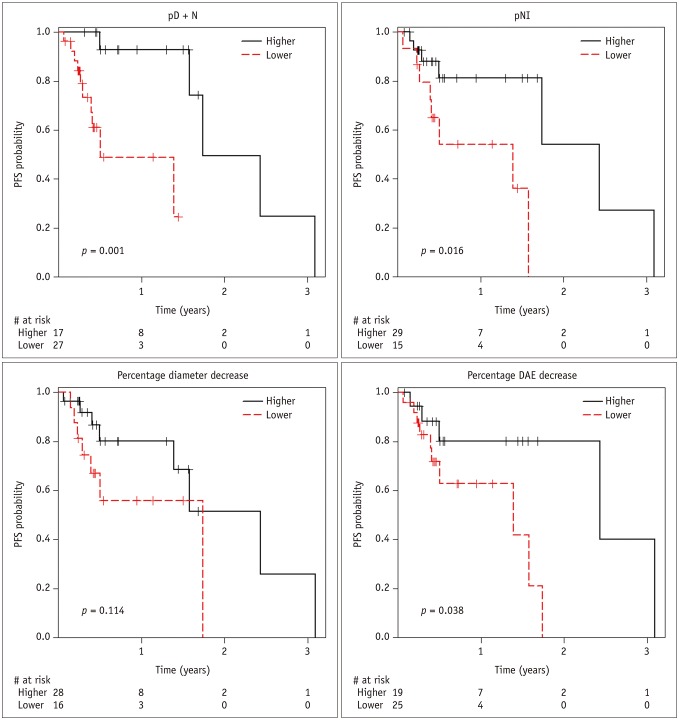
Post-TARE HCC with pD + N ≥ 35.5% showed significantly longer PFS than those with pD + N < 35.5% (p = 0.001). The local tumor progression hazard ratio was 17.3 (p = 0.009) for pD + N < 35.5% versus pD + N ≥ 35.5% groups. HCCs with a high pNI tended to have longer PFS, although this difference did not reach statistical significance.
CONCLUSION
HCCs with a larger pD + N are less likely to develop local progression after TARE.
Keyword
MeSH Terms
Figure
Reference
-
1. Kennedy A, Coldwell D, Sangro B, Wasan H, Salem R. Integrating radioembolization ((90)Y microspheres) into current treatment options for liver tumors: introduction to the international working group report. Am J Clin Oncol. 2012; 35:81–90. PMID: 20938320.2. Kennedy A, Nag S, Salem R, Murthy R, McEwan AJ, Nutting C, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys. 2007; 68:13–23. PMID: 17448867.
Article3. Sangro B, Iñarrairaegui M, Bilbao JI. Radioembolization for hepatocellular carcinoma. J Hepatol. 2012; 56:464–473. PMID: 21816126.
Article4. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92:205–216. PMID: 10655437.5. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010; 30:52–60. PMID: 20175033.
Article6. Keppke AL, Salem R, Reddy D, Huang J, Jin J, Larson AC, et al. Imaging of hepatocellular carcinoma after treatment with yttrium-90 microspheres. AJR Am J Roentgenol. 2007; 188:768–775. PMID: 17312067.
Article7. Riaz A, Kulik L, Lewandowski RJ, Ryu RK, Giakoumis Spear G, Mulcahy MF, et al. Radiologic-pathologic correlation of hepatocellular carcinoma treated with internal radiation using yttrium-90 microspheres. Hepatology. 2009; 49:1185–1193. PMID: 19133645.
Article8. Herfarth KK, Hof H, Bahner ML, Lohr F, Höss A, van Kaick G, et al. Assessment of focal liver reaction by multiphasic CT after stereotactic single-dose radiotherapy of liver tumors. Int J Radiat Oncol Biol Phys. 2003; 57:444–451. PMID: 12957256.
Article9. Maturen KE, Feng MU, Wasnik AP, Azar SF, Appelman HD, Francis IR, et al. Imaging effects of radiation therapy in the abdomen and pelvis: evaluating "innocent bystander" tissues. Radiographics. 2013; 33:599–619. PMID: 23479716.
Article10. Gulec SA, Mesoloras G, Stabin M. Dosimetric techniques in 90Y-microsphere therapy of liver cancer: the MIRD equations for dose calculations. J Nucl Med. 2006; 47:1209–1211. PMID: 16818957.11. Tsuji Y, Takahashi N, Fletcher JG, Hough DM, McMenomy BP, Lewis DM, et al. Subtraction color map of contrast-enhanced and unenhanced CT for the prediction of pancreatic necrosis in early stage of acute pancreatitis. AJR Am J Roentgenol. 2014; 202:W349–W356. PMID: 24660733.
Article12. Prionas ND, Lindfors KK, Ray S, Huang SY, Beckett LA, Monsky WL, et al. Contrast-enhanced dedicated breast CT: initial clinical experience. Radiology. 2010; 256:714–723. PMID: 20720067.
Article13. Contal C, O'Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Computational Statistics & Data Analysis. 1999; 30:253–270.
Article14. Chen BB, Hsu CY, Yu CW, Hou HA, Liu CY, Wei SY, et al. Dynamic contrast-enhanced MR imaging measurement of vertebral bone marrow perfusion may be indicator of outcome of acute myeloid leukemia patients in remission. Radiology. 2011; 258:821–831. PMID: 21212370.
Article15. Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979; 86:420–428. PMID: 18839484.
Article16. Büsing KA, Kilian AK, Schaible T, Debus A, Weiss C, Neff KW. Reliability and validity of MR image lung volume measurement in fetuses with congenital diaphragmatic hernia and in vitro lung models. Radiology. 2008; 246:553–561. PMID: 18055874.
Article17. Wong CY, Salem R, Raman S, Gates VL, Dworkin HJ. Evaluating 90Y-glass microsphere treatment response of unresectable colorectal liver metastases by [18F]FDG PET: a comparison with CT or MRI. Eur J Nucl Med Mol Imaging. 2002; 29:815–820. PMID: 12029557.18. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–247. PMID: 19097774.
Article19. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981; 47:207–214. PMID: 7459811.
Article20. Reiner CS, Morsbach F, Sah BR, Puippe G, Schaefer N, Pfammatter T, et al. Early treatment response evaluation after yttrium-90 radioembolization of liver malignancy with CT perfusion. J Vasc Interv Radiol. 2014; 25:747–759. PMID: 24630751.
Article21. Shim JH, Lee HC, Kim SO, Shin YM, Kim KM, Lim YS, et al. Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? A validation study of old and new models. Radiology. 2012; 262:708–718. PMID: 22187634.
Article22. Li Z, Bonekamp S, Halappa VG, Corona-Villalobos CP, Pawlik T, Bhagat N, et al. Islet cell liver metastases: assessment of volumetric early response with functional MR imaging after transarterial chemoembolization. Radiology. 2012; 264:97–109. PMID: 22627602.
Article23. Gowdra Halappa V, Corona-Villalobos CP, Bonekamp S, Li Z, Reyes D, Cosgrove D, et al. Neuroendocrine liver metastasis treated by using intraarterial therapy: volumetric functional imaging biomarkers of early tumor response and survival. Radiology. 2013; 266:502–513. PMID: 23192780.
Article24. Miller FH, Keppke AL, Reddy D, Huang J, Jin J, Mulcahy MF, et al. Response of liver metastases after treatment with yttrium-90 microspheres: role of size, necrosis, and PET. AJR Am J Roentgenol. 2007; 188:776–783. PMID: 17312068.
Article25. Arslanoglu A, Chalian H, Sodagari F, Seyal AR, Töre HG, Salem R, et al. Threshold for enhancement in treated hepatocellular carcinoma on MDCT: effect on necrosis quantification. AJR Am J Roentgenol. 2016; 206:536–543. PMID: 26901009.
Article26. Blazic IM, Lilic GB, Gajic MM. Quantitative assessment of rectal cancer response to neoadjuvant combined chemotherapy and radiation therapy: comparison of three methods of positioning region of interest for ADC measurements at diffusion-weighted MR imaging. Radiology. 2017; 282:418–428. PMID: 27253423.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Radioembolization for hepatocellular carcinoma: what clinicians need to know
- Radioembolization for the treatment of hepatocellular carcinoma
- Role of Yttrium-90 Radioembolization in the Management of Hepatocellular Carcinoma
- Gastrectomy for the treatment of refractory gastric ulceration after radioembolization with 90Y microspheres
- Advanced Stage Hepatocellular Carcinoma Successfully Treated with Transarterial Radioembolization and Multi-tyrosine Kinase Inhibitor Therapy