Clin Endosc.
2019 Jan;52(1):15-20. 10.5946/ce.2018.193.
Risk Factors for Lymph Node Metastasis in Undifferentiated-Type Gastric Carcinoma
- Affiliations
-
- 1Center for Gastric Cancer, National Cancer Center, Goyang, Korea. mckook@ncc.re.kr
- KMID: 2438139
- DOI: http://doi.org/10.5946/ce.2018.193
Abstract
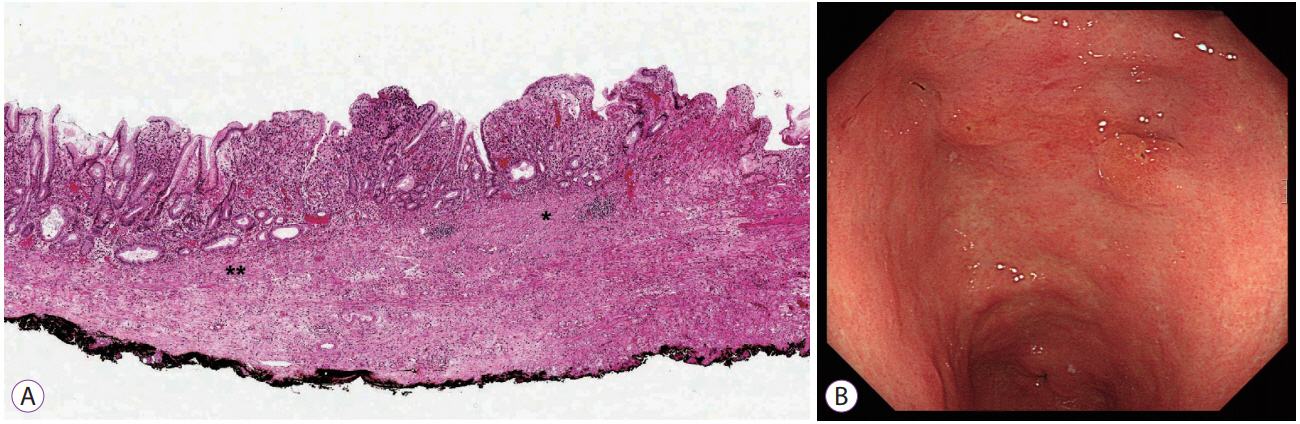
- Undifferentiated-type carcinoma has a high incidence of lymph node metastasis. The independent risk factors for lymph node metastasis in undifferentiated-type carcinoma are invasion depth, tumor size, lymphovascular invasion, and presence of ulcer. In the cases that meet the curative resection criteria, no lymph node metastasis was observed in the Japanese studies, but some metastases were observed in Korean studies. After performing curative endoscopic submucosal dissection, the survival rate is similar to that of gastrectomy. The discrepancy between endoscopy and pathology is high in undifferentiated-type carcinoma. The tumor size in endoscopy is a significant risk factor for non-curative resection, and when the tumor size is small, the non-curative resection rate is significantly reduced. Lymphovascular invasion can be assessed in pathologic examination and D2-40 stain is helpful. The presence of ulcer should be determined by pathology, but ulcer's omission in pathology report makes the analysis difficult. Undifferentiatedtype carcinomas with differentiated-type components show higher lymph node metastasis rate than that of pure undifferentiatedtype carcinomas. The lymph node metastasis rate of signet ring cell type is lower than that of other undifferentiated-type carcinomas and is similar to differentiated-type carcinomas. The application of these additional histologic findings may improve the indication of endoscopic submucosal dissection.
MeSH Terms
Figure
Reference
-
1. Sugano H, Nakamura K, Kato Y. Pathological studies of human gastric cancer. Acta Pathol Jpn. 1982; 32 Suppl 2:329–347.2. Choi KK, Bae JM, Kim SM, et al. The risk of lymph node metastases in 3951 surgically resected mucosal gastric cancers: implications for endoscopic resection. Gastrointest Endosc. 2016; 83:896–901.
Article3. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011; 14:113–123.4. Yamao T, Shirao K, Ono H, et al. Risk factors for lymph node metastasis from intramucosal gastric carcinoma. Cancer. 1996; 77:602–606.
Article5. Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000; 3:219–225.
Article6. Seto Y, Shimoyama S, Kitayama J, et al. Lymph node metastasis and preoperative diagnosis of depth of invasion in early gastric cancer. Gastric Cancer. 2001; 4:34–38.
Article7. Chung JW, Jung HY, Choi KD, et al. Extended indication of endoscopic resection for mucosal early gastric cancer: analysis of a single center experience. J Gastroenterol Hepatol. 2011; 26:884–887.
Article8. Ye BD, Kim SG, Lee JY, et al. Predictive factors for lymph node metastasis and endoscopic treatment strategies for undifferentiated early gastric cancer. J Gastroenterol Hepatol. 2008; 23:46–50.
Article9. Hirasawa T, Gotoda T, Miyata S, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer. 2009; 12:148–152.
Article10. Kunisaki C, Takahashi M, Nagahori Y, et al. Risk factors for lymph node metastasis in histologically poorly differentiated type early gastric cancer. Endoscopy. 2009; 41:498–503.
Article11. Li C, Kim S, Lai JF, et al. Risk factors for lymph node metastasis in undifferentiated early gastric cancer. Ann Surg Oncol. 2008; 15:764–769.
Article12. Abe S, Oda I, Suzuki H, et al. Short- and long-term outcomes of endoscopic submucosal dissection for undifferentiated early gastric cancer. Endoscopy. 2013; 45:703–707.
Article13. Kim JH, Kim YH, Jung DH, et al. Follow-up outcomes of endoscopic resection for early gastric cancer with undifferentiated histology. Surg Endosc. 2014; 28:2627–2633.
Article14. Oka S, Tanaka S, Higashiyama M, et al. Clinical validity of the expanded criteria for endoscopic resection of undifferentiated-type early gastric cancer based on long-term outcomes. Surg Endosc. 2014; 28:639–647.
Article15. Ahn JY, Park HJ, Park YS, et al. Endoscopic resection for undifferentiated-type early gastric cancer: immediate endoscopic outcomes and longterm survivals. Dig Dis Sci. 2016; 61:1158–1164.
Article16. Park JC, Lee YK, Kim SY, et al. Long-term outcomes of endoscopic submucosal dissection in comparison to surgery in undifferentiated-type intramucosal gastric cancer using propensity score analysis. Surg Endosc. 2018; 32:2046–2057.
Article17. Lee S, Choi KD, Han M, et al. Long-term outcomes of endoscopic submucosal dissection versus surgery in early gastric cancer meeting expanded indication including undifferentiated-type tumors: a criteria-based analysis. Gastric Cancer. 2018; 21:490–499.
Article18. Takizawa K, Takashima A, Kimura A, et al. A phase II clinical trial of endoscopic submucosal dissection for early gastric cancer of undifferentiated type: Japan Clinical Oncology Group study JCOG1009/1010. Jpn J Clin Oncol. 2013; 43:87–91.
Article19. Kim EH, Park JC, Song IJ, et al. Prediction model for non-curative resection of endoscopic submucosal dissection in patients with early gastric cancer. Gastrointest Endosc. 2017; 85:976–983.
Article20. Kim JM, Sohn JH, Cho MY, et al. Pre- and post-ESD discrepancies in clinicopathologic criteria in early gastric cancer: the NECA-Korea ESD for early gastric cancer prospective study (N-Keep). Gastric Cancer. 2016; 19:1104–1113.
Article21. Kim SG, Park CM, Lee NR, et al. Long-term clinical outcomes of endoscopic submucosal dissection in patients with early gastric cancer: a prospective multicenter cohort study. Gut Liver. 2018; 12:402–410.
Article22. Hasuike N, Ono H, Boku N, et al. A non-randomized confirmatory trial of an expanded indication for endoscopic submucosal dissection for intestinal-type gastric cancer (cT1a): the Japan Clinical Oncology Group study (JCOG0607). Gastric Cancer. 2018; 21:114–123.
Article23. Kwak DS, Min YW, Lee JH, et al. Outcomes of endoscopic submucosal dissection for early gastric cancer with undifferentiated-type histology: a clinical simulation using a non-selected surgical cohort. Gut Liver. 2018; 12:263–270.
Article24. Okada K, Fujisaki J, Yoshida T, et al. Long-term outcomes of endoscopic submucosal dissection for undifferentiated-type early gastric cancer. Endoscopy. 2012; 44:122–127.
Article25. Yamamoto Y, Fujisaki J, Hirasawa T, et al. Therapeutic outcomes of endoscopic submucosal dissection of undifferentiated-type intramucosal gastric cancer without ulceration and preoperatively diagnosed as 20 millimetres or less in diameter. Dig Endosc. 2010; 22:112–118.
Article26. Yonemura Y, Endou Y, Tabachi K, et al. Evaluation of lymphatic invasion in primary gastric cancer by a new monoclonal antibody, D2-40. Hum Pathol. 2006; 37:1193–1199.
Article27. Ordóñez NG. Podoplanin: a novel diagnostic immunohistochemical marker. Adv Anat Pathol. 2006; 13:83–88.
Article28. Pusztaszeri MP, Seelentag W, Bosman FT. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem. 2006; 54:385–395.
Article29. Kim WH, Park CK, Kim YB, et al. A standardized pathology report for gastric cancer. Korean J Pathol. 2005; 39:106–113.30. Fujimoto A, Ishikawa Y, Akishima-Fukasawa Y, et al. Significance of lymphatic invasion on regional lymph node metastasis in early gastric cancer using LYVE-1 immunohistochemical analysis. Am J Clin Pathol. 2007; 127:82–88.
Article31. Park SM, Kim BW, Kim JS, Kim YW, Kim GJ, Ryu SJ. Can endoscopic ulcerations in early gastric cancer be clearly defined before endoscopic resection? A survey among endoscopists. Clin Endosc. 2017; 50:473–478.
Article32. Hanaoka N, Tanabe S, Mikami T, Okayasu I, Saigenji K. Mixed-histologic-type submucosal invasive gastric cancer as a risk factor for lymph node metastasis: feasibility of endoscopic submucosal dissection. Endoscopy. 2009; 41:427–432.
Article33. Takizawa K, Ono H, Kakushima N, et al. Risk of lymph node metastases from intramucosal gastric cancer in relation to histological types: how to manage the mixed histological type for endoscopic submucosal dissection. Gastric Cancer. 2013; 16:531–536.
Article34. Kwon KJ, Shim KN, Song EM, et al. Clinicopathological characteristics and prognosis of signet ring cell carcinoma of the stomach. Gastric Cancer. 2014; 17:43–53.
Article35. Kim BS, Oh ST, Yook JH, Kim BS. Signet ring cell type and other histologic types: differing clinical course and prognosis in T1 gastric cancer. Surgery. 2014; 155:1030–1035.
Article36. Ha TK, An JY, Youn HK, Noh JH, Sohn TS, Kim S. Indication for endoscopic mucosal resection in early signet ring cell gastric cancer. Ann Surg Oncol. 2008; 15:508–513.
Article37. Tong JH, Sun Z, Wang ZN, et al. Early gastric cancer with signet-ring cell histologic type: risk factors of lymph node metastasis and indications of endoscopic surgery. Surgery. 2011; 149:356–363.
Article38. Guo CG, Zhao DB, Liu Q, et al. Risk factors for lymph node metastasis in early gastric cancer with signet ring cell carcinoma. J Gastrointest Surg. 2015; 19:1958–1965.
Article39. Bang CS, Park JM, Baik GH, et al. Therapeutic outcomes of endoscopic resection of early gastric cancer with undifferentiated-type histology: a Korean ESD registry database analysis. Clin Endosc. 2017; 50:569–577.
Article40. Mariette C, Carneiro F, Grabsch HI, van der Post RS, Allum W, de Manzoni G. Consensus on the pathological definition and classification of poorly cohesive gastric carcinoma. Gastric Cancer. 2019; 22:1–9.
Article41. Kwon CH, Kim YK, Lee S, et al. Gastric poorly cohesive carcinoma: a correlative study of mutational signatures and prognostic significance based on histopathological subtypes. Histopathology. 2018; 72:556–568.
Article42. Kim YH, Park JH, Park CK, et al. Histologic purity of signet ring cell carcinoma is a favorable risk factor for lymph node metastasis in poorly cohesive, submucosa-invasive early gastric carcinoma. Gastric Cancer. 2017; 20:583–590.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Prognosis of Patients with Stage IV Gastric Carcinoma without Distant Metastasis
- Risk Factors Affecting Lymph Node Metastasis and Recurrence in Early Gastric Cancer
- Survival of Node-Positive Mucosal Gastric Carcinoma Patients
- Analysis of Clinicopathological Factors Associated with Lymph Node Metastasis in Early Gastric Cancer Review of 2,137 cases
- Endoscopic Resection of Undifferentiated Early Gastric Cancer