Cancer Res Treat.
2019 Jan;51(1):378-390. 10.4143/crt.2018.070.
Plasma Macrophage Migration Inhibitory Factor and CCL3 as Potential Biomarkers for Distinguishing Patients with Nasopharyngeal Carcinoma from High-Risk Individuals Who Have Positive Epstein-Barr Virus Capsid Antigen-Specific IgA
- Affiliations
-
- 1Department of Clinical Laboratory, Affiliated Tumor Hospital of Zhengzhou University, Henan Tumor Hospital, Zhengzhou, China.
- 2Department of Clinical Laboratory, Sun Yat-Sen University Cancer Center, Guangzhou, China. liuwl@sysucc.org.cn
- 3State Key Laboratory of Oncology in Southern China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China. zengmsh@sysucc.org.cn
- KMID: 2437628
- DOI: http://doi.org/10.4143/crt.2018.070
Abstract
- PURPOSE
The purpose of this study was to identify novel plasma biomarkers for distinguishing nasopharyngeal carcinoma (NPC) patients from healthy individuals who have positive Epstein-Barr virus (EBV) viral capsid antigen (VCA-IgA).
MATERIALS AND METHODS
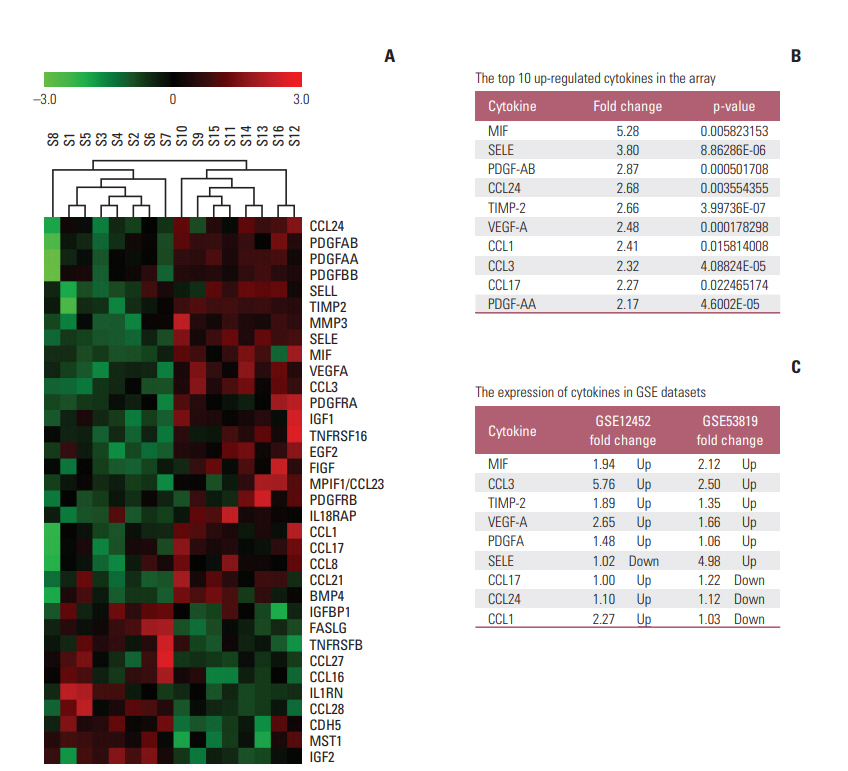
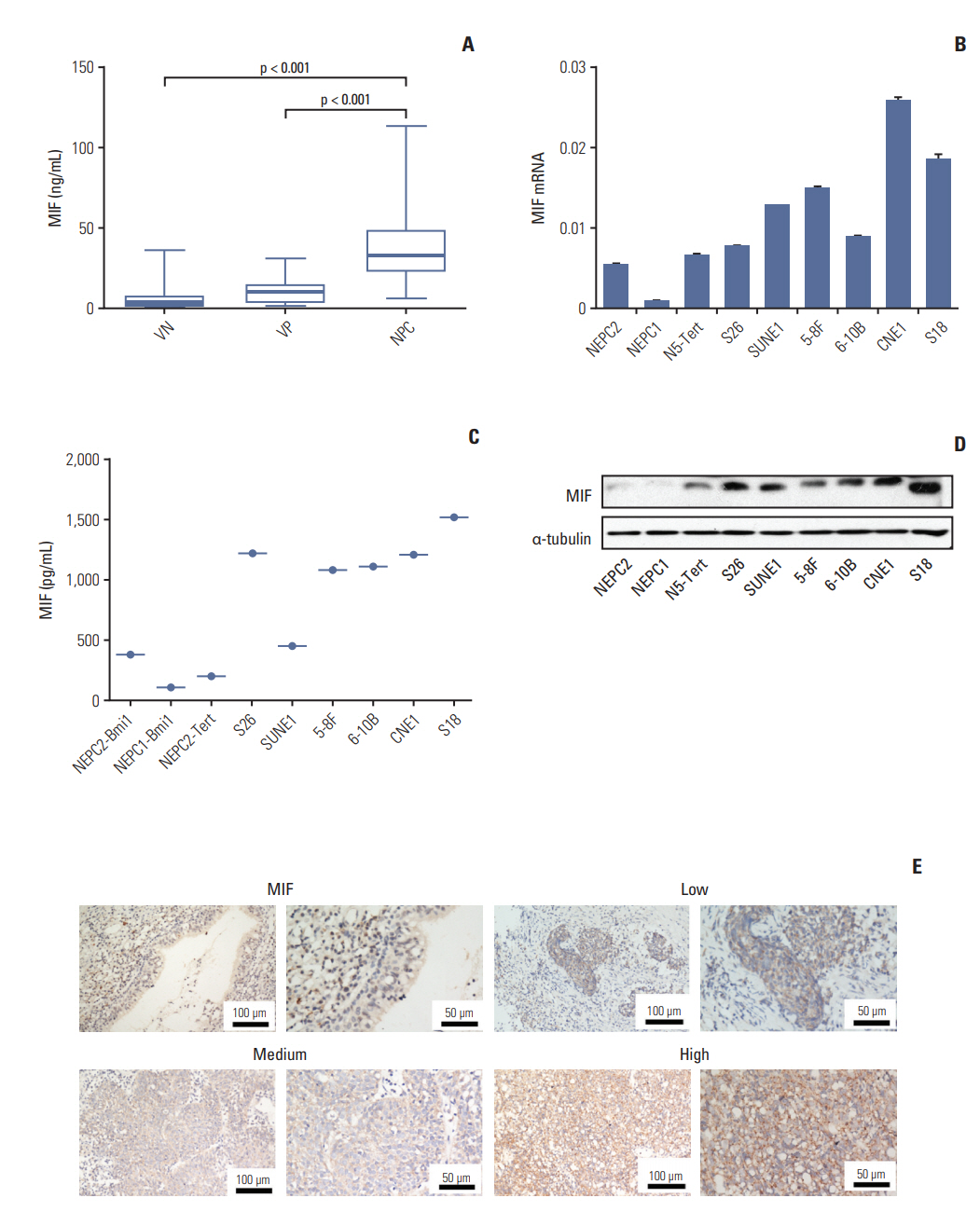
One hundred seventy-four plasma cytokines were analyzed by a Cytokine Array in eight healthy individuals with positive EBV VCA-IgA and eight patients with NPC. Real-time polymerase chain reaction, Western blotting, enzyme-linked immunosorbent assay (ELISA), and immunohistochemistry were employed to detect the expression levels of macrophage migration inhibitory factor (MIF) and CC chemokine ligand 3 (CCL3) in NPC cell lines and tumor tissues. Plasma MIF and CCL3 were measured by ELISA in 138 NPC patients, 127 EBV VCA-IgA negative (VN) and 100 EBV VCA-IgA positive healthy donors (VP). Plasma EBV VCA-IgA was determined by immunoenzymatic techniques.
RESULTS
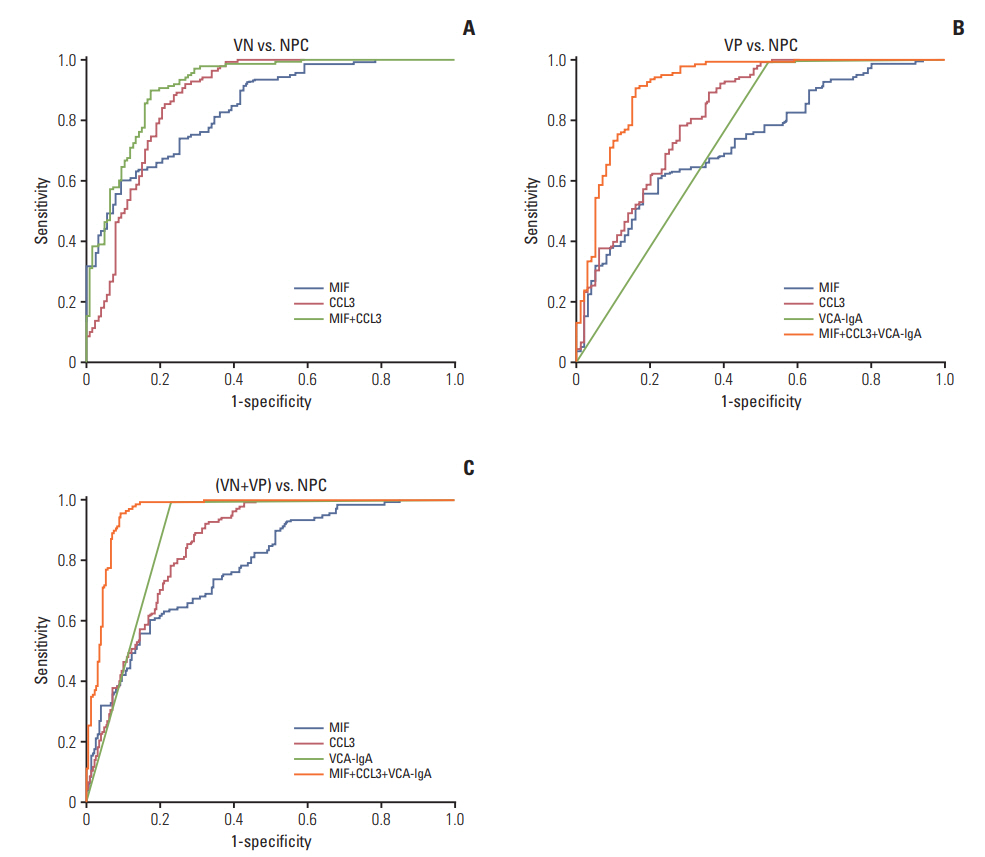
Thirty-four of the 174 cytokines varied significantly between the VP and NPC group. Plasma MIF and CCL3 were significantly elevated in NPC patients compared with VN and VP. Combination of MIF and CCL3 could be used for the differential diagnosis of NPC from VN cohort (area under the curve [AUC], 0.913; sensitivity, 90.00%; specificity, 80.30%), and combination of MIF, CCL3, and VCA-IgA could be used for the differential diagnosis of NPC from VP cohort (AUC, 0.920; sensitivity, 90.00%; specificity, 84.00%), from (VN+VP) cohort (AUC, 0.961; sensitivity, 90.00%; specificity, 92.00%). Overexpressions of MIF and CCL3 were observed in NPC plasma, NPC cell lines and NPC tissues.
CONCLUSION
Plasma MIF, CCL3, and VCA-IgA combination significantly improves the diagnostic specificity of NPC in high-risk individuals.
Keyword
MeSH Terms
-
Biomarkers*
Blotting, Western
Capsid*
Cell Line
Chemokine CCL3
Cohort Studies
Cytokines
Diagnosis
Diagnosis, Differential
Enzyme-Linked Immunosorbent Assay
Herpesvirus 4, Human*
Humans
Immunoglobulin A*
Immunohistochemistry
Macrophages*
Plasma*
Real-Time Polymerase Chain Reaction
Sensitivity and Specificity
Tissue Donors
Biomarkers
Chemokine CCL3
Cytokines
Immunoglobulin A
Figure
Reference
-
References
1. Razak AR, Siu LL, Liu FF, Ito E, O'Sullivan B, Chan K. Nasopharyngeal carcinoma: the next challenges. Eur J Cancer. 2010; 46:1967–78.
Article2. Cao SM, Guo X, Li NW, Xiang YQ, Hong MH, Min HQ. Clinical analysis of 1,142 hospitalized cantonese patients with nasopharyngeal carcinoma. Ai Zheng. 2006; 25:204–8.3. Chen CJ, Liang KY, Chang YS, Wang YF, Hsieh T, Hsu MM, et al. Multiple risk factors of nasopharyngeal carcinoma: Epstein-Barr virus, malarial infection, cigarette smoking and familial tendency. Anticancer Res. 1990; 10:547–53.4. Liu JP, Cassar L, Pinto A, Li H. Mechanisms of cell immortalization mediated by EB viral activation of telomerase in nasopharyngeal carcinoma. Cell Res. 2006; 16:809–17.
Article5. Lo KW, To KF, Huang DP. Focus on nasopharyngeal carcinoma. Cancer Cell. 2004; 5:423–8.
Article6. Karray H, Ayadi W, Fki L, Hammami A, Daoud J, Drira MM, et al. Comparison of three different serological techniques for primary diagnosis and monitoring of nasopharyngeal carcinoma in two age groups from Tunisia. J Med Virol. 2005; 75:593–602.
Article7. Neel HB 3rd, Pearson GR, Taylor WF. Antibodies to Epstein-Barr virus in patients with nasopharyngeal carcinoma and in comparison groups. Ann Otol Rhinol Laryngol. 1984; 93(5 Pt 1):477–82.
Article8. Yi Z, Yuxi L, Chunren L, Sanwen C, Jihneng W, Jisong Z, et al. Application of an immunoenzymatic method and an immunoautoradiographic method for a mass survey of nasopharyngeal carcinoma. Intervirology. 1980; 13:162–8.
Article9. Xia C, Zhu K, Zheng G. Expression of EBV antibody EA-IgA, Rta-IgG and VCA-IgA and SA in serum and the implication of combined assay in nasopharyngeal carcinoma diagnosis. Int J Clin Exp Pathol. 2015; 8:16104–10.10. Tay JK, Chan SH, Lim CM, Siow CH, Goh HL, Loh KS. The role of Epstein-Barr virus DNA load and serology as screening tools for nasopharyngeal carcinoma. Otolaryngol Head Neck Surg. 2016; 155:274–80.
Article11. Chan KC. Plasma Epstein-Barr virus DNA as a biomarker for nasopharyngeal carcinoma. Chin J Cancer. 2014; 33:598–603.
Article12. Chang KP, Hao SP, Chang JH, Wu CC, Tsang NM, Lee YS, et al. Macrophage inflammatory protein-3alpha is a novel serum marker for nasopharyngeal carcinoma detection and prediction of treatment outcomes. Clin Cancer Res. 2008; 14:6979–87.13. Hsin LJ, Kao HK, Chen IH, Tsang NM, Hsu CL, Liu SC, et al. Serum CXCL9 levels are associated with tumor progression and treatment outcome in patients with nasopharyngeal carcinoma. PLoS One. 2013; 8:e80052.
Article14. MacBeath G. Protein microarrays and proteomics. Nat Genet. 2002; 32 Suppl:526–32.
Article15. Li J, Mo HY, Xiong G, Zhang L, He J, Huang ZF, et al. Tumor microenvironment macrophage inhibitory factor directs the accumulation of interleukin-17-producing tumor-infiltrating lymphocytes and predicts favorable survival in nasopharyngeal carcinoma patients. J Biol Chem. 2012; 287:35484–95.
Article16. Lee H, Rhee H, Kang HJ, Kim HS, Min BS, Kim NK, et al. Macrophage migration inhibitory factor may be used as an early diagnostic marker in colorectal carcinomas. Am J Clin Pathol. 2008; 129:772–9.
Article17. Gamez-Pozo A, Sanchez-Navarro I, Calvo E, Agullo-Ortuno MT, Lopez-Vacas R, Diaz E, et al. PTRF/cavin-1 and MIF proteins are identified as non-small cell lung cancer biomarkers by label-free proteomics. PLoS One. 2012; 7:e33752.
Article18. Menten P, Wuyts A, Van Damme J. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev. 2002; 13:455–81.
Article19. Blunt MD, Koehrer S, Dobson RC, Larrayoz M, Wilmore S, Hayman A, et al. The dual Syk/JAK inhibitor cerdulatinib antagonizes B-cell receptor and microenvironmental signaling in chronic lymphocytic leukemia. Clin Cancer Res. 2017; 23:2313–24.
Article20. Wu Y, Li YY, Matsushima K, Baba T, Mukaida N. CCL3-CCR5 axis regulates intratumoral accumulation of leukocytes and fibroblasts and promotes angiogenesis in murine lung metastasis process. J Immunol. 2008; 181:6384–93.
Article21. Xiong D, Du Y, Wang HB, Zhao B, Zhang H, Li Y, et al. Non-muscle myosin heavy chain IIA mediates Epstein-Barr virus infection of nasopharyngeal epithelial cells. Proc Natl Acad Sci U S A. 2015; 112:11036–41.
Article22. Tsao SW, Yip YL, Tsang CM, Pang PS, Lau VM, Zhang G, et al. Etiological factors of nasopharyngeal carcinoma. Oral Oncol. 2014; 50:330–8.
Article23. Bach JP, Rinn B, Meyer B, Dodel R, Bacher M. Role of MIF in inflammation and tumorigenesis. Oncology. 2008; 75:127–33.
Article24. Liao B, Zhong BL, Li Z, Tian XY, Li Y, Li B. Macrophage migration inhibitory factor contributes angiogenesis by up-regulating IL-8 and correlates with poor prognosis of patients with primary nasopharyngeal carcinoma. J Surg Oncol. 2010; 102:844–51.
Article25. Meyer-Siegler KL, Leifheit EC, Vera PL. Inhibition of macrophage migration inhibitory factor decreases proliferation and cytokine expression in bladder cancer cells. BMC Cancer. 2004; 4:34.
Article26. DE Souza MB, Curioni OA, Kanda JL, DE Carvalho MB. Serum and salivary macrophage migration inhibitory factor in patients with oral squamous cell carcinoma. Oncol Lett. 2014; 8:2267–75.
Article27. Maurer M, von Stebut E. Macrophage inflammatory protein-1. Int J Biochem Cell Biol. 2004; 36:1882–6.
Article28. Horuk R. Development and evaluation of pharmacological agents targeting chemokine receptors. Methods. 2003; 29:369–75.
Article29. Yuan Y, Liu J, Liu Z, He Y, Zhang Z, Jiang C, et al. Chemokine CCL3 facilitates the migration of hepatoma cells by changing the concentration intracellular Ca. Hepatol Res. 2010; 40:424–31.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Epstein-Barr Virus Infection Presented as Evans Syndrome
- Prognostic Value of Serum Epstein-Barr Virus Antibodies and Their Correlation with TNM Classification in Patients with Locoregionally Advanced Nasopharyngeal Carcinoma
- A Case of Epstein-Barr Virus-Related Dacryoadenitis
- Epstein-Barr Virus Infection with Acute Pancreatitis Associated with Cholestatic Hepatitis
- A Case of Epstein-Barr Virus-associated Brainstem Encephalitis Presenting with Sixth Cranial Nerve Palsy in a Child